39 mole mass conversions worksheet answers
Chapter 4 assignments. Chemistry conversion worksheet answers 1 6. This page includes measurement worksheets for length area angles volume capacity mass time and temperature in metric u s. Conversion worksheet answer sheet. The resources here cater to the learning requirements of grade 3 grade 4 grade 5 and grade 6. Period date si units.
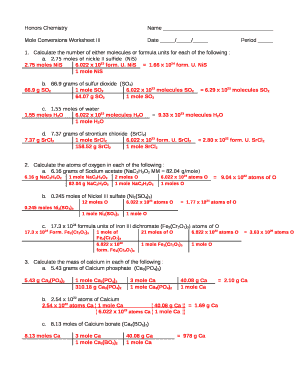
(ANSWER 386.3g of LiNO3) 4) Using the following equation: Fe2O3 + 3 H2 -----> 2 Fe + 3 H2O . Calculate how many grams of iron can be made from 16.5 grams of Fe2O3 by the following equation. Worksheet for Basic Stoichiometry. Part 1: Mole ←→ Mass Conversions. Convert the following number of moles of chemical into its corresponding mass in grams.
3 c-What is the mass of 1.40x1020 atoms of calcium? 1.40 x 1020 atoms Ca 1 mol Ca 40.08 g Ca= .00692 g Ca 6.022 x 1023 atoms Ca 1 mol Ca d-Calculate the mass in grams of one calcium atom. 1atom Ca 1 mol Ca 40.08 g Ca= 6.7 x 10-23 g Ca 6.022 x 1023 atoms Ca 1 mol Ca 12. a-How many atoms are contained in 28.0 grams of nitrogen?

Mole mass conversions worksheet answers
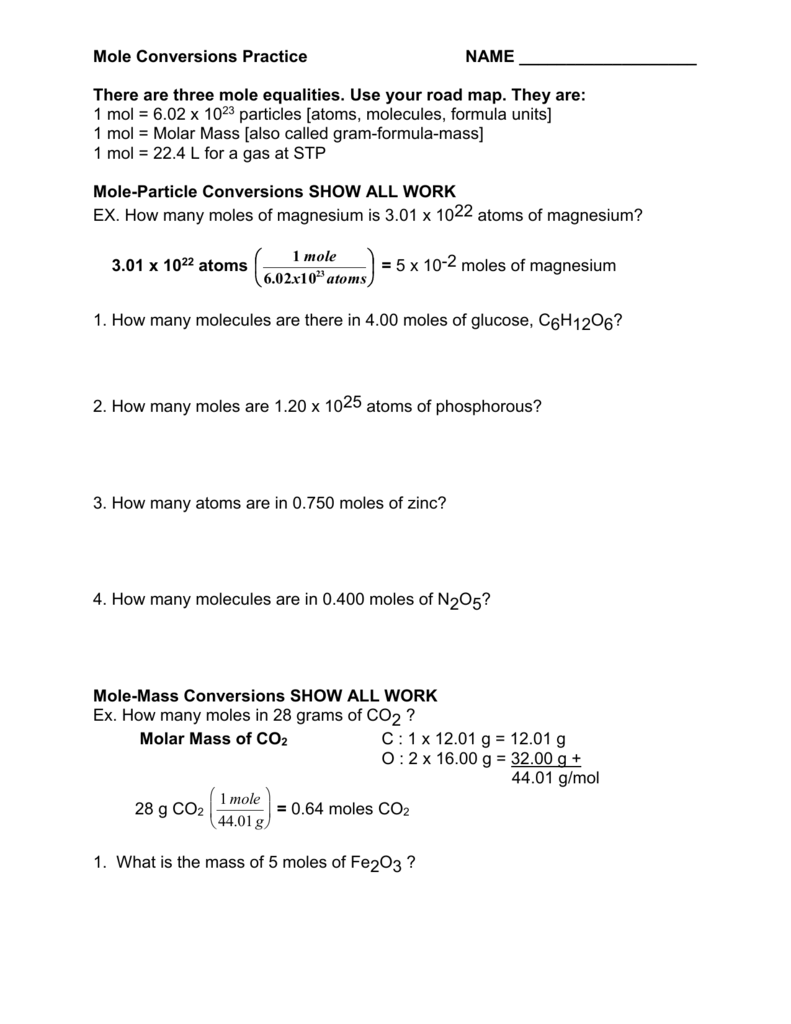
name: suggested answers date: _____ mole conversions - practice problems It is important to be able to convert between units of mass and volume measurement and 'moles.' The mole is a unit for counting atoms and molecules representing 602,000,000,000,000,000,000,000 particles. This is often written as
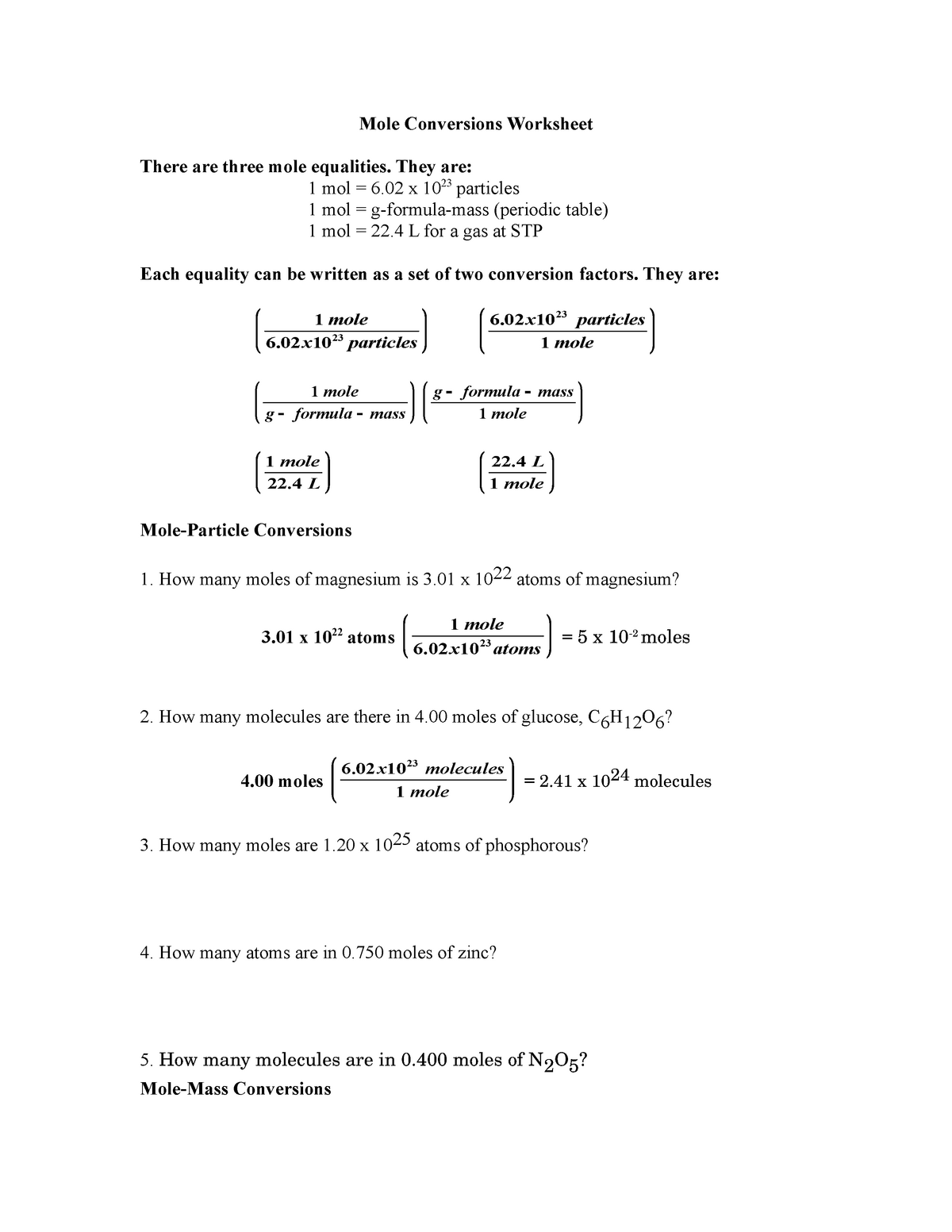
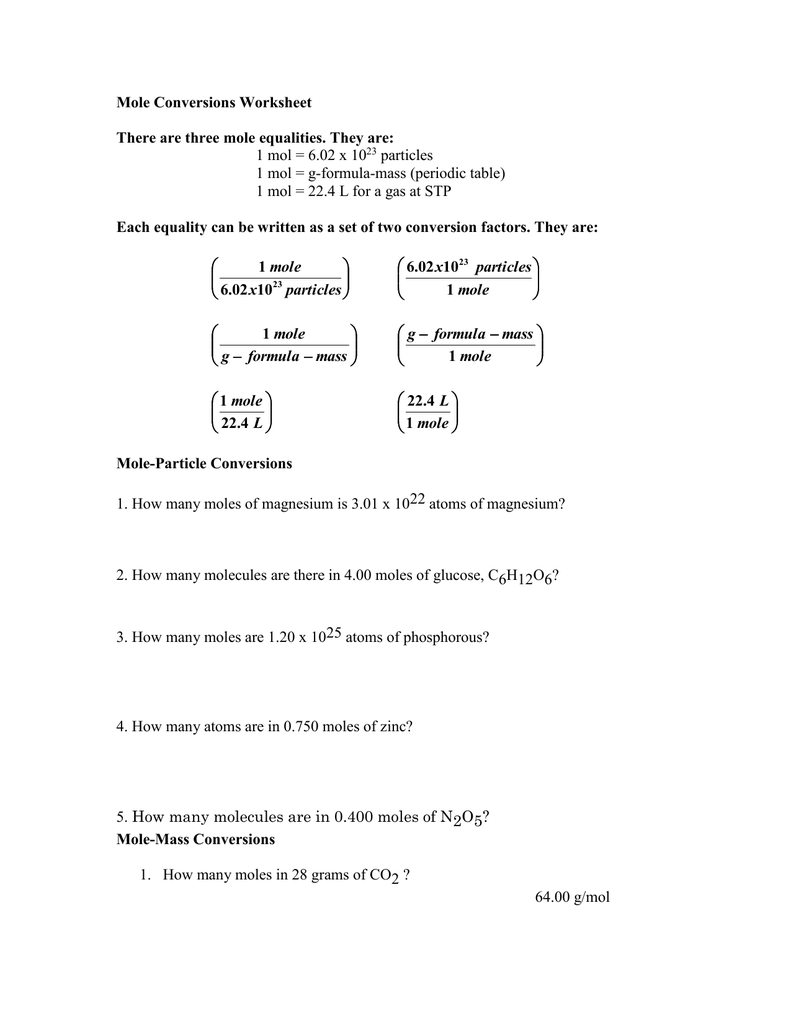
Mole Conversions Worksheet Working with Moles and Particles There are three mole equalities. They are: 1 mol = 6.02 x 1023 particles (atom, molecule or ion) 1 mol = gram formula mass of a substance 1 mol = 22.4 L at STP The equality for moles and particles can be written as a set of two conversion factors: 1 mole 6.02x1023 particles OR
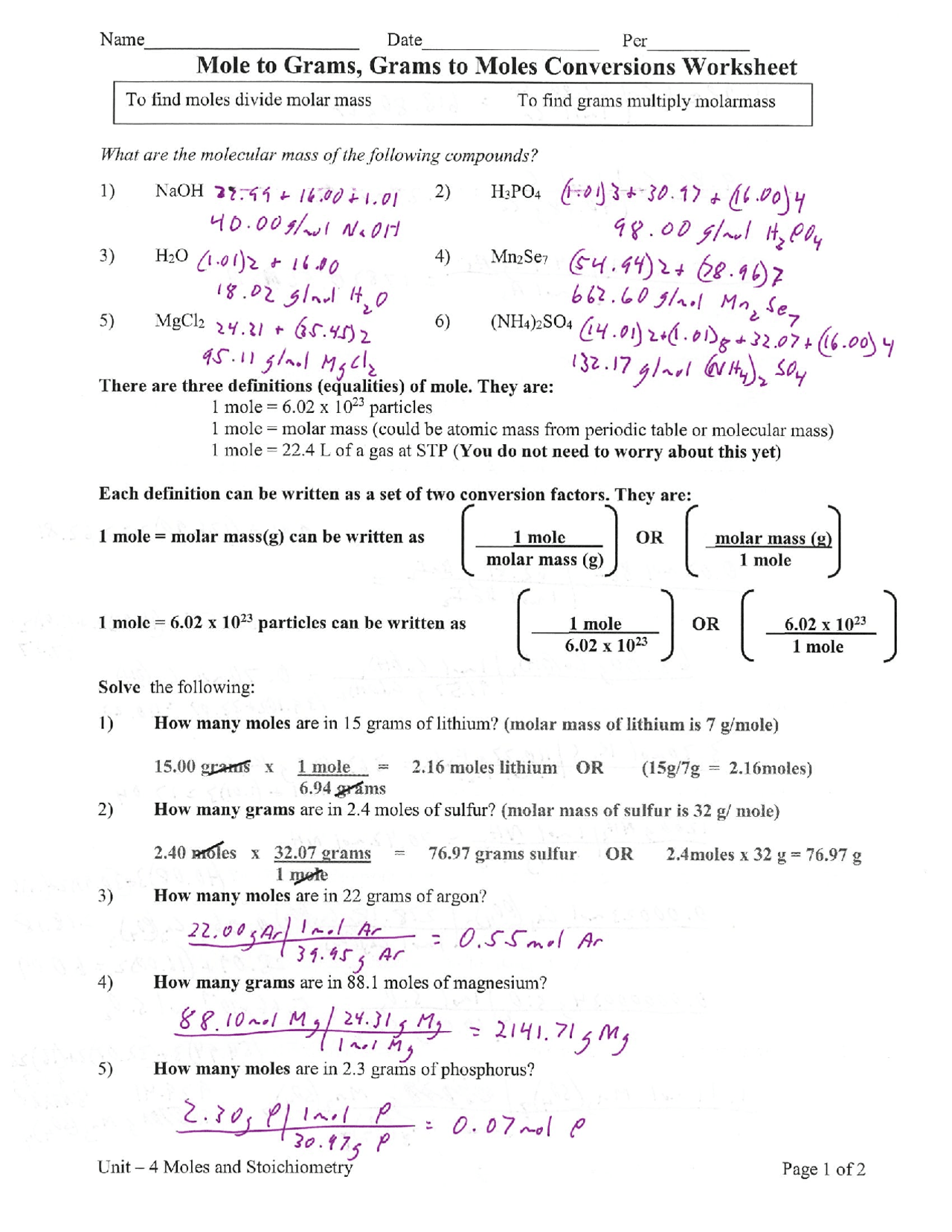
Mole to Grams, Grams to Moles Conversions Worksheet What are the molecular weights of the following compounds? 1) NaOH 2) H 3PO 4 3) H 2O 4) Mn 2Se 7 5) MgCl 2 6) (NH 4) 2SO 4 There are three definitions (equalities) of mole. They are: 1 mole = 6.02 x 1023 particles 1 mole = molar mass (could be atomic mass from periodic table or molecular mass)
Mole mass conversions worksheet answers.
The mass of 11 mol of hydrogen chloride. The number of moles of 99.4 grams of NaCl. Expert Level (hint you must use both equations) The molarity when 54.8 grams of lithium sulfate are dissolved to make 250 mL of solution. The molarity when 99.1 grams of (NH 4)2SO4 are dissolved to make 0.5 L of solution.
How to calculate the molar mass of a compound: •For elements, the molar mass is the same thing as the atomic mass. •For chemical compounds, it's the sum of the masses of all of the atoms in the molecule. •Example: CO 2 C: 12.01 grams x 1 atom = 12.01 grams O: 16.00 grams x 2 atom = 32.00 grams Total: 1mole of CO 2 = 44.01 grams
Mole Conversion Problems Note: Some of these are the same compounds as in the "Molar Mass" worksheet, so you can use the formula weights from that worksheet as the starting point for your calculations. 1. How many moles are in 72.9g of HCl? 2. How many moles are in 79.85g Fe 2O 3 3. How many moles are in 11.2' of CO 2 gas at S.T.P.? 4.
Mole calculation worksheet answer key. 1 mole 6 02 x 1023particles 1 mole molar mass could be atomic mass from periodic table or molecular mass 1 mole 22 4 l of a gas at stp you do not need to worry about this yet each definition can be written as a set of two conversion factors.
Mass-Mole Conversions Worksheet Molar mass of a substance = mass of one mole of the substance One mole of an element = the atomic mass of that element (from the periodic table) One mole of a compound = the sum of the atomic masses of the atoms present in the compound The units of molar mass are always grams per mole (g/mol).
Moles, Mass and Particles Worksheet - Answer Key 1) 1.3 x 1023 formula units 2) 1.91 x 1024 formula units 3) 4.1 x 102 g 4) 2.1 x 102 g 5) 1.2 x 102 g 6) 3.92 x 1023 formula units 7) 3.1 x 102 g 8) 1.07 x 1024 formula units 9) 1.7 x 1022 formula units 10) 4.3 x 102 g 11) 7.82 x 1023 molecules 12) 200 g 13) 17.1 g 14) 1.33 x 1023 formula units ...
Created Date: 2/23/2015 4:14:14 PM
Worksheet 1 1.2 Mole Conversions Worksheet There are three mole equalities: 23 1 mol 6.02 x 10 particles 1 mol = molar mass 1 mol = 22.4 L (for a gas at STP)
Worksheet - Mole Conversions Name: Show all work utilizing dimensional analysis wherever possible. Include all units and account for significant figures. A. What is the molar mass of: (make sure to show clearly defined and complete work here) 1. H2 2. Ba(OH)2. 3. CO 4. NH4Cl. 5. NiSO4 6. Al(NO3)3. Conversion between moles and mass
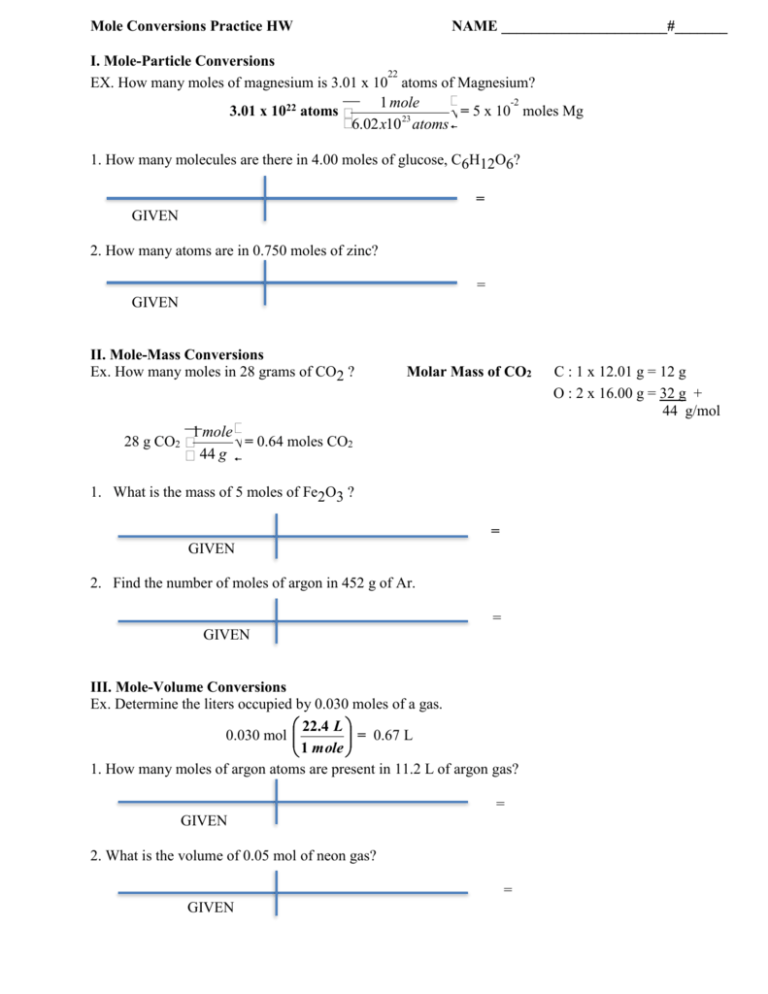
Mole Calculation Worksheet W 340 Everett Community College Tutoring Center Student Support Services Program 1) How many moles are in 40.0 grams of water? 2) How many grams are in 3.7 moles of Na 2 O? 3) How many atoms are in 14 moles of cadmium?
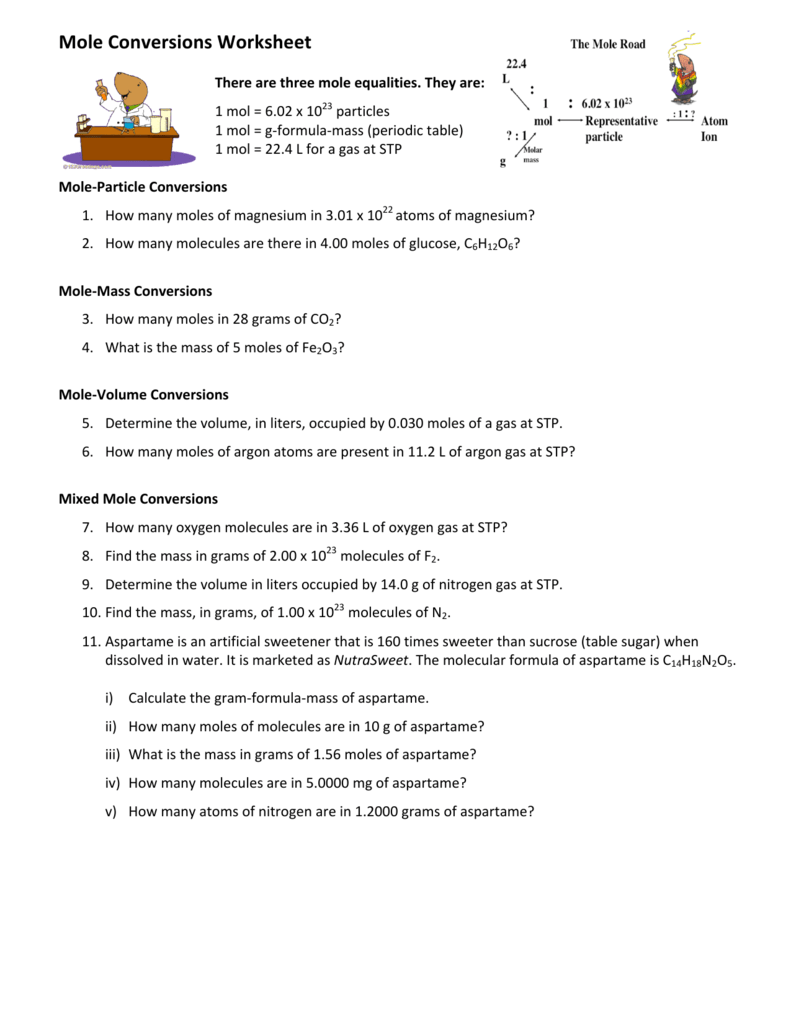
Find the mass, in grams, of 1.00 x 1023 molecules of N 2. 5. Aspartame is an artificial sweetener that is 160 times sweeter than sucrose (table sugar) when dissolved in water.
Solutions to the Molar Mass Practice Worksheet: Important note to students: All of the units given here are "grams per mole", which may be ... Also, remember that if you don't use units in your answer, the answer is wrong! All answers are rounded to the nearest 0.1 grams. 1) 102.9 g/mol 2) 303.3 g/mol 3) 74.1 g/mol 4) 164.0 g/mol 5) 96.0 ...
Moles and mass worksheet. 1 mole 6 02 x 1023particles 1 mole molar mass could be atomic mass from periodic table or molecular mass 1 mole 22 4 l of a gas at stp you do not need to worry about this yet each definition can be written as a set of two conversion factors. Lots of grams per mole. 1 mole 6 02 x 1023 particles. View us version.
Mole Conversions Worksheet. There are three mole equalities. They are: 1 mol = 6 x 10. 23 particles. 1 mol = g-formula-mass (periodic table) 1 mol = 22 L for a gas at STP. Each equality can be written as a set of two conversion factors. They are: x particles. mole. 23 02 10. 1 mole. x particles. 1. 02 10. 23 g formula mass. 1 mole mole. g ...
3. Add it all together and put units of grams/mole after the number. Example: Find the molar mass of sodium carbonate, Na 2CO 3. Na 2 x 23.0 = 46.0 C 1 x 12.0 = 12.0 O 3 x 16.0 = 48.0 molar mass = 106.0 g/mole For many (but not all) problems, you can simply round the atomic weights and the molar mass to the nearest 0.1 g/mole.
Mole Conversions Worksheet Working with Moles and Particles There are three mole equalities. They are: 1 mol = 6.02 x 1023 particles (atom, molecule or ion) 1 mol = gram formula mass of a substance 1 mol = 22.4 L for a gas at STP
Molecular Formula Worksheet ANSWER KEY. Write the molecular formulas of the following compounds: 1) A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole. C4O2H8. 2) A compound with an empirical formula of C4H4O and a molar mass of 136 grams per mole. C8H8O2
32.066 g of sulfur 6.022 1023 sulfur atoms 1 mol of sulfur General Plan for Converting Mass, Amount, and Numbers of Particles Use Avogadro's number for conversion. Mass of substance Amount of substance in moles Number of atoms, molecules, or formula units of substance Convert using the molar mass of the substance. 1 2 3 Name Class Date Problem ...
Molar Conversion Worksheet Hint:-Start with the given value-Set up a conversion factor-Make sure your units are canceling 1. What is the mass of 1 mole of Barium acetate, Ba(C2H3O2)2? 2. What is the molar mass (g/mol) of cyclohexanol, C6H11OH? 3. How many moles are in 2.35 g of H2O? 4. If we have 0.072 g of FeCl3 then how many moles are there? 5.
Answer 3.71 g Al Conversions like this are possible for any substance, as long as the proper atomic mass, formula mass, or molar mass is known (or can be determined) and expressed in grams per mole. Figure \(\PageIndex{1}\) is a chart for determining what conversion factor is needed, and Figure \(\PageIndex{2}\) is a flow diagram for the steps ...
Mole Conversions Worksheet #1 1. Mole -> Mass Conversions - using molar mass of each substance, convert the following quantities. a. 10.0 mol Cr 520 g f. 0.160 mol H 2 O 2.88 g b. 3.32 mol K 130 g g. 5.08 mol Ca(NO 3) 2 834 g c. 2.20 x 10-3 mol Sn 0.261 g h. 15.0 mol H 2 SO 4 1470 g d. 0.720 mol Be 6.48 g i. ...
Mole worksheet use two decimal places for the molar masses and report your answer to the correct number of significant figures. How many moles is 12 5 g of magnesium hydroxide 214 moles 2. 22 4 g co 8. Molar mass worksheet answer key calculate the molar masses of the following chemicals. 2 07 x 10 atoms p 7. 1 x 40 1 40 1 n. 6 x 16 0 96 0.
1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 L of a gas at STP (You do not need to worry about this yet) Each definition can be written as a set of two conversion factors. They are: 1 mole = molar mass(g) can be written as ____1 mole OR _molar mass (g) molar mass (g) 1 mole

















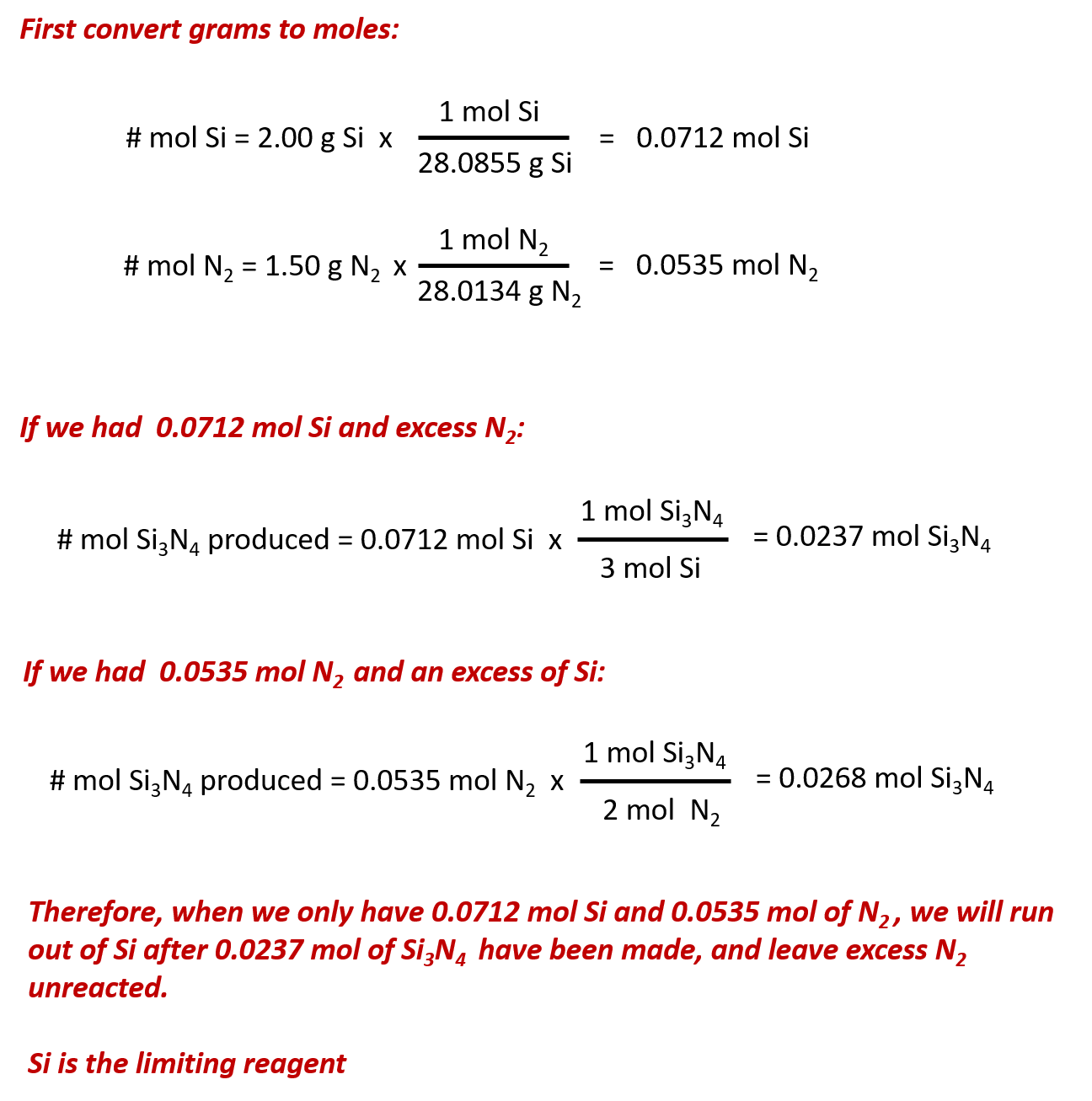







0 Response to "39 mole mass conversions worksheet answers"
Post a Comment