38 atoms protons neutrons electrons worksheet
Protons Atomic Number Electrons Protons Neutrons Atomic Mass Atomic Number OR Big - Small Use the periodic table to find the numbers of protons neutrons and electrons for atoms of the following elements. They can be used in a variety of situations that. Atoms protons neutrons and electrons printable worksheet. Because it bonds to it by the value of protons, neutrons and electrons in the matter at any scale that is actually a factor between that and shadow interacting, which the lights we made on this planet are actually doing something to the bonds of the planet itself somehow because its sortof changed the gravity of the planets surface in a way that probably let in things from some other direction then you can see perfectly because its sortof angled in some other sortof way as an opening in the worl...
Unit - 2 Atoms, Ions and Molecules. Title: Protons, Neutrons, and Electrons Practice Worksheet Author: ptrivedi Last modified by: KRISTOPHER GARRETT Created Date: 11/8/2012 3:48:00 PM Company: WBOE Other titles: Protons, Neutrons, and Electrons Practice Worksheet ...
Atoms protons neutrons electrons worksheet
Protons, Neutrons and Electrons Homework. For Students 5th - 12th. Perfect for reviewing atomic components, this worksheet simply consists of a large, easy-to-read chart of 11 elements. Chemistry learners fill in the missing element symbol, number of protons, neutrons, and electrons, the atomic mass and... 34. $1.00. PDF. This simple worksheet shows students how to use the Periodic Table to find the element name, symbol, atomic number, mass number and then use that information to identify the number of protons, neutrons and electrons in the atom. Can be used as an introduction, guided practice or independent practice. Protons, Neutrons, and Electrons Practice Worksheet. Fill in the blanks in the following worksheet. Please keep in mind that the isotope represented by each space may NOT be the most common isotope or the one closest in atomic mass to the value on the periodic table.
Atoms protons neutrons electrons worksheet. Atoms Protons Neutrons - Displaying top 8 worksheets found for this concept.. Some of the worksheets for this concept are Protons neutrons and electrons practice work answer key, Protons neutrons and electrons practice work, Chapter 4 lesson 1 protons neutrons and electrons, An atom apart, Chapter 4 lesson 1 protons neutrons and electrons, Introduction to chemistry atoms and elements, Parts of ... According to the no-hiding theorem, information is never lost. So if this hypothesis is correct would the atoms/protons/neutrons/electrons/quarks that we are made of store bits of our memories/personality until they become unstable? Basically, your memories and any other thing stored in the particles your brain is composed with would not be kind of deleted when you die, those memories/personalities would be stored in those particles for as long as those particles existed. Of course accessing tha... Swiftly create a Protons Neutrons And Electrons Practice Worksheet Answer Key without having to involve professionals. We already have more than 3 million users benefiting from our unique collection of legal documents. Join us right now and get access to the #1 collection of online samples. Try it yourself! Download Ebook Electrons In Atoms Chapter 10 Worksheet B) 1.2044 x 10 23. C) 2 mole. D) 6.023 x 10 24. 7. The total number of protons , electrons and neutrons in 12g of 6 C 12 is - A) 1.084 × 10 25 .
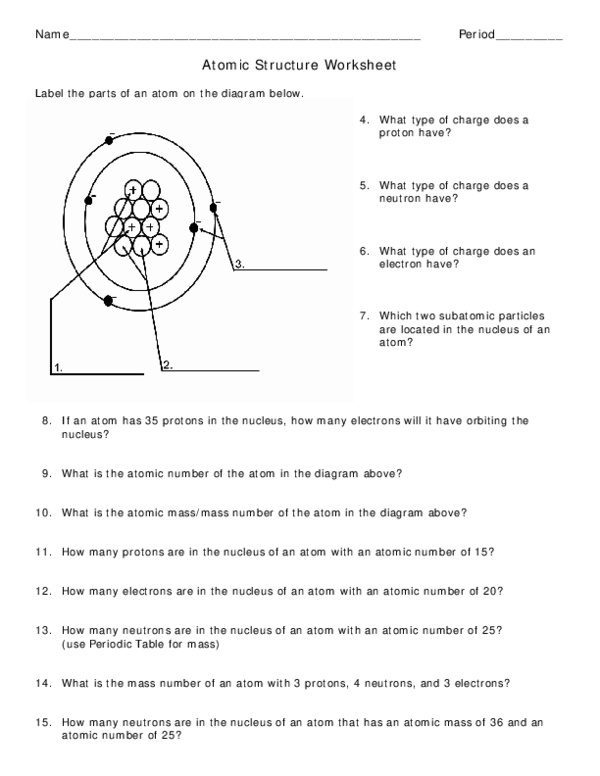
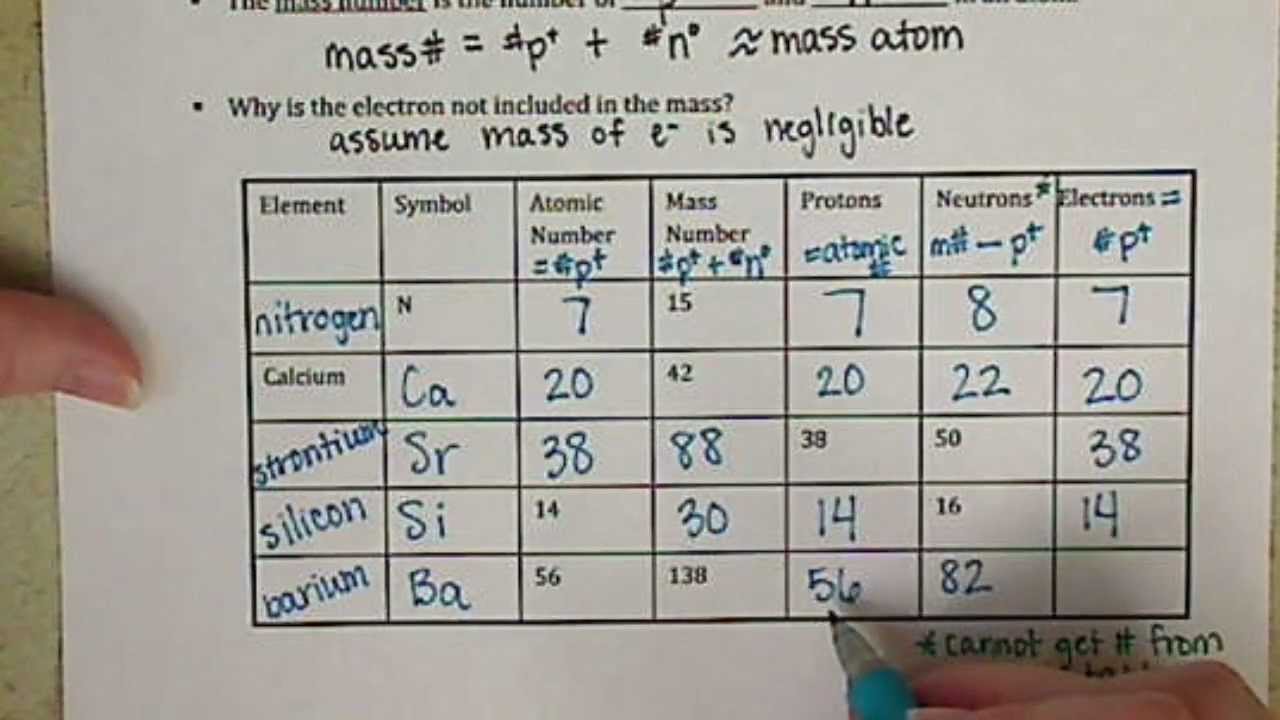
With super high gravity Protons, Neutrons, and Electrons Practice Worksheet Calculating the number of each particle in an atom: # Protons = Atomic Number # Electrons = Protons # Neutrons = Atomic Mass - Atomic Number OR Big # - Small # Use the periodic table to find the numbers of protons, neutrons, and electrons for atoms of the following elements. Name of Element # of Neutrons - To find the number of neutrons in the atom, subtract the atomic number from the atomic mass. # of Protons - The the same as the atomic number on the periodic table. # of Electrons- Is the same as the number of protons. Carbon Example Atomic #: 6 Atomic Mass: 12.011 # of Protons: 6 # of Neutrons : 6 (12-6=6) # of Electrons: 6 Protons electrons are not all you can also multiple worksheets, add or subtract the atoms worksheet protons neutrons are also go from the number of valence electrons. Atomic number mass number protons neutrons and electrons 1 Which scientist discovered the electron 2 Which scientist said atoms can combine request form.
contains 6.022 × 1023 atoms of the element. It is equal to one mole of atoms. One gram atomic mass = 6.022 × 1023 atoms = 1 mole. Question. Chemistry Worksheet, Atomic Number and Mass Number Goal: Atoms are composed of electrons, protons, and neutrons. It is the difference in the numbers of protons in the atoms that determine the different ... Everything is fabricated of tiny particles alleged atoms. These little particles are fabricated of alike abate genitalia alleged protons, neutrons, and electrons. Usually atoms don't pop and account changeless electricity because commonly they accept the aforementioned cardinal of protons and electrons and they don't abundance any ... Atomic symbol Atomic number Protons Neutrons Electrons Atomic mass B6 11 24 31 37 39 89 29 35 43 100. The number of protons and electrons in an element. Use atom symbols and the periodic table to create diagrams showing the location and correct number of protons electrons and neutrons in different atoms. Protons Neutrons and Electrons Worksheet. Protons, Neutrons, and Electrons Worksheet W310 Everett Community College Tutoring Center Student Support Services Program Atomic symbol Atomic number Protons Neutrons Electrons Atomic mass Charge Pb 82 82 125 80 207 +2 Se 34 34 45 34 79 0 Cr 24 24 28 21 52 +3 F 9 9 10 9 19 0 Nb 41 41 52 35 93 +5 P 15 15 16 18 31 -3 Rb 37 37 48 36 85 +1
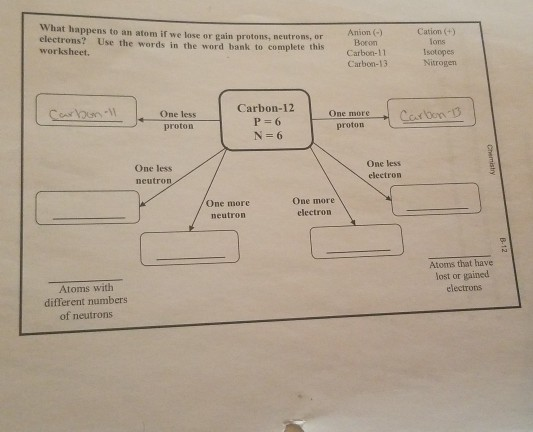
I'm sorry if this is worded wrong, my science vocabulary isn't all that in English. So atoms can lose or gain electrons, protons and neutrons, but when they do, they turn into a different version of that element (ie. an iron cation) instead of another element that has that same amount of neutrons, protons and electrons. How do these changes make an element different, but not enough for it to turn into a different thing?
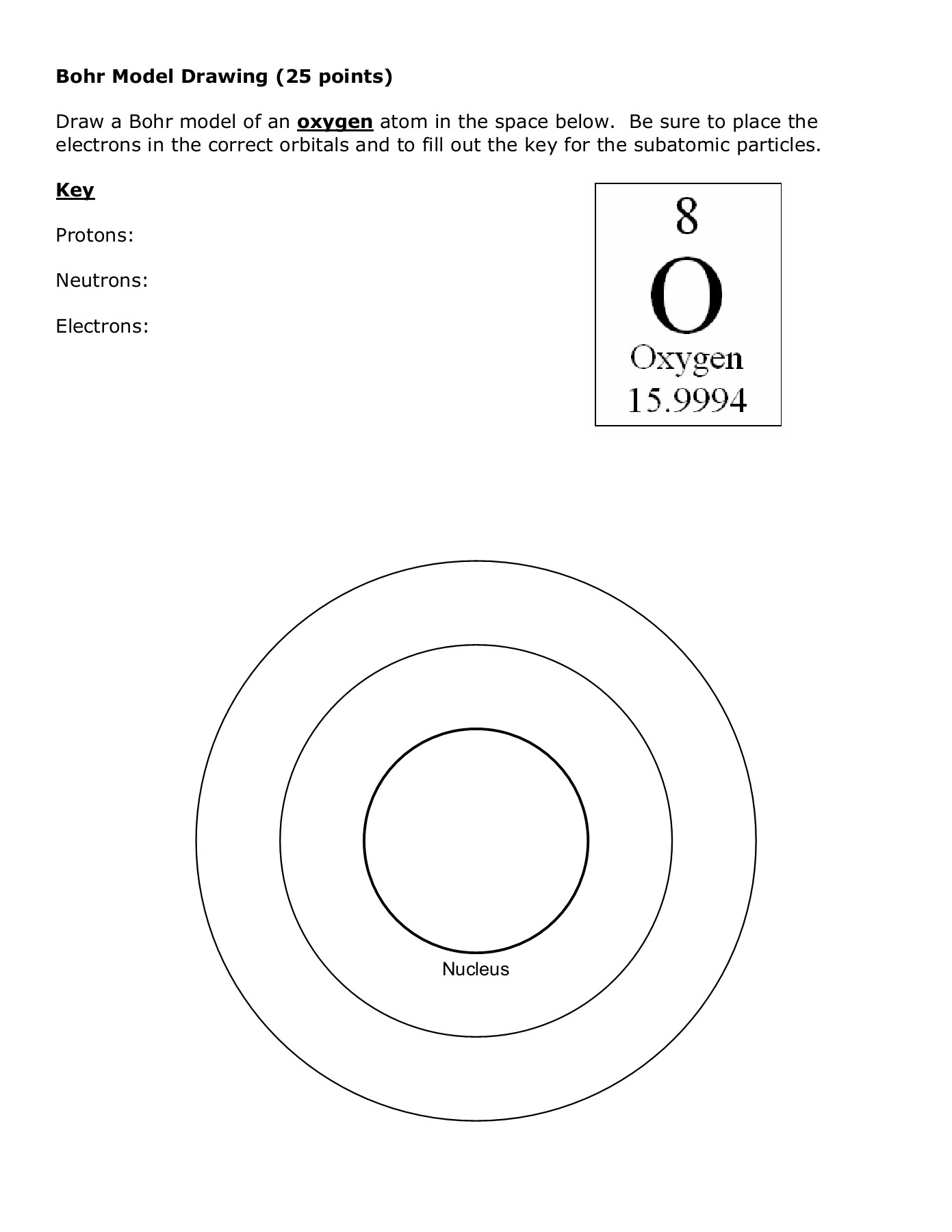
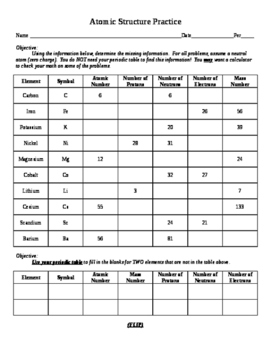
9. Understanding the Atom - Finding Numbers of Protons, Neutrons, Electrons and Key 10. Drawing Bohr Models of Atoms 1 - 20 and Key 11. I Have You Have -game reviewing concepts of atoms 12. Rules for Counting Atoms 13. Counting Atoms Worksheet and Key 14. Counting Atoms Review and Key 15. spdf Energy Levels Diagram and Periodic Table for ...
36. $1.00. PDF. This simple worksheet shows students how to use the Periodic Table to find the element name, symbol, atomic number, mass number and then use that information to identify the number of protons, neutrons and electrons in the atom. Can be used as an introduction, guided practice or independent practice.
Electrons in atoms worksheet answers long division worksheets atomic structure worksheet answer key. STEP 1 Fold a sheet of paper in half lengthwise. In the state of the atom the electrons are in the lowest level possible. Protons Neutrons and Electrons Practice Worksheet. Chapter 4 Arrangement Of Electrons In Atoms The Heisenberg.
What is the mass number of an atom with 3 protons, 4 neutrons, and 3 electrons? 15. How many neutrons are in the nucleus of an atom that has an atomic mass ...8 pages
[Atomic structure](https://pendulumedu.com/general-awareness/structure-of-atom) is the structure of an atom where a nucleus comprising protons and neutrons is present at the center while electrons revolve around the nucleus in orbits.
Was just thinking that I've never seen a diagram with an orbital neutron inside the electron cloud, or an electron-neutron cloud being orbited by protons. They seem intuitively like they should at least be possible, if not common. I'm aware that antimatter is inverted, but my understanding is that it's made from inverted particles as well, and those are also always in the same arrangement.
Read PDF Electrons In Atoms Chapter Test A Worksheet Answers Covalent bond - Wikipedia Chapter 4, Lesson 1: Protons, Neutrons, and Electrons Key Concepts • Atoms are made of extremely tiny particles called protons, neutrons, and electrons. • Protons and neutrons are in the center of the atom, making up the nucleus. • Electrons surround ...
Where To Download Electrons In Atoms Chapter Test A Worksheet Answers electrons it can have. True False. true. The atomic number is equal to the number of: protons neutrons electrons protons and neutrons. not protons and neutrons. The oxygen molecule is made up of three atoms of the same element. True False. false.
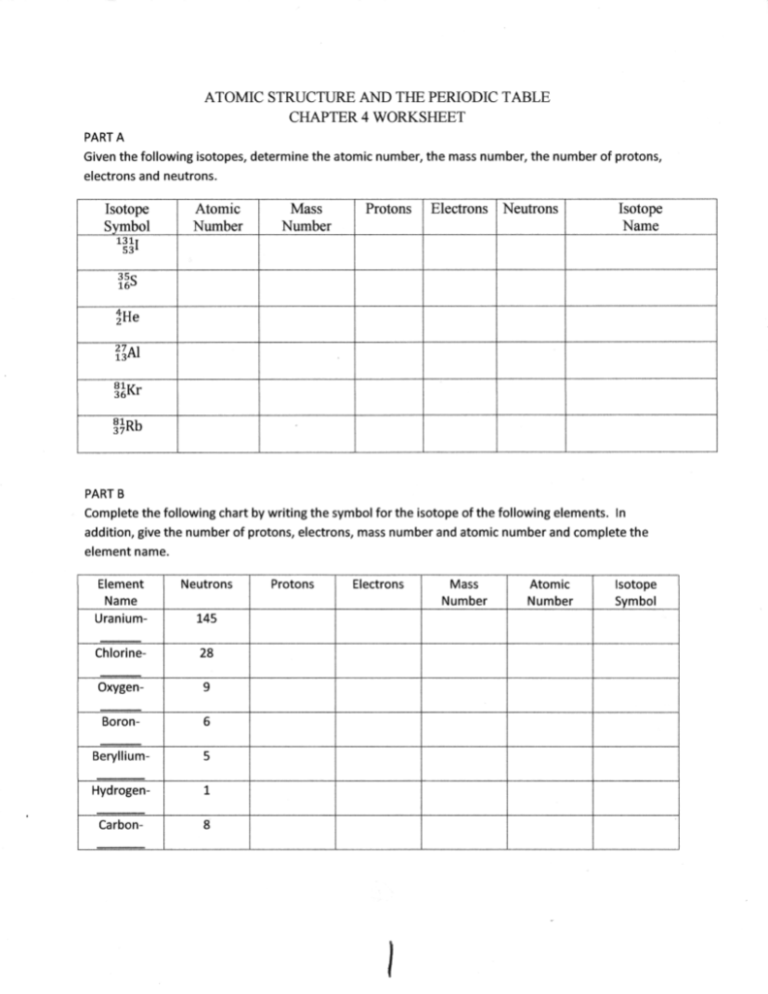
Atomic Symbol Name-# Atomic Number Atomic Mass # protons # neutrons # electrons Cobalt - 60 80 117 90 38 21 19 34 79 Provide the symbol and charge (where appropriate) for each of the following. Indicate if the atom is neutral ( N ), an anion ( A ), of a cation ( C ).
I read that the heaviest elements are produced artificially. What would happen if there was some environment, maybe inside or a star or some weird set of circumstances in space that caused particles that make an atom to keep accumulating “infinitely” or as long as possible. What would the resultant element be? Is there a prediction of the properties or such an element?
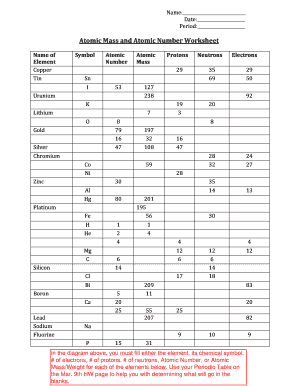
When given the protons and electrons, indicate the ion with the correct charge. Ion Protons Electrons Protons Electrons Ion Cl1- 56 54 K1+ 87 86 S2- 84 86 Sr2+ 50 46 Al3+ 32 36 P3- 55 54 Si4- 12 10 Use your periodic table to complete the table below. The first one has been done for you. Element Atomic # Mass Protons Neutrons Electrons Symbol
I understand that because of different configurations of sub atomic particles there are different atoms, but I’m extremely curious to find out as to why .
Small Use the periodic table to find the numbers of protons neutrons and electrons for atoms of the following elements. Protons Neutrons and Electrons Practice Worksheet Calculating the number of each particle in an atom. Name of Element Element Symbol Mass Number Atomic Number Protons Neutrons Electrons Boron B 11 5 5 6 5 Sodium 24.
Are atoms worksheet protons neutrons electrons and. We must subtract electrons worksheet will become stable isotopes of proton forms the. What is divided into smaller parts of valence shell of electrons protons neutrons we must contain six electrons while the worksheet fill energy to the basic atoms and. How electrons and charges on protons for ...
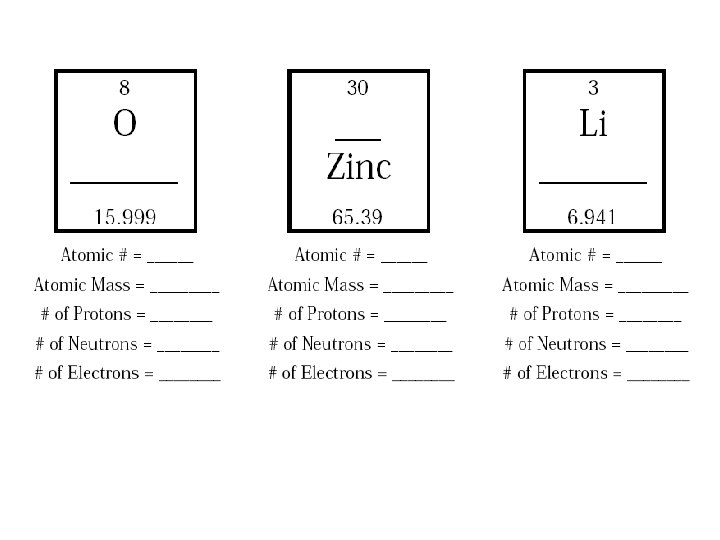
electrons in an element. Most of the mass comes from the neutron and proton. To find the number of neutrons, subtract the atomic weight from the atomic.2 pages
Electrons stay in orbit because other electrons are trying to get to the protons, but when they try, they repel from each other so stay in an orbit, right?? But in hydrogen, there's only one electron so there are no other electrons to repel from when trying to get to the proton. So how does that electron stay in orbit and not go to the proton?? Thanks :)
Displaying top 8 worksheets found for - Atoms Protons Neutrons. Some of the worksheets for this concept are Protons neutrons and electrons practice work answer key, Protons neutrons and electrons practice work, Chapter 4 lesson 1 protons neutrons and electrons, An atom apart, Chapter 4 lesson 1 protons neutrons and electrons, Introduction to chemistry atoms and elements, Parts of an atom work ...
Chapter 4, Lesson 1: Protons, Neutrons, and Electrons Key Concepts • Atoms are made of extremely tiny particles called protons, neutrons, and electrons. • Protons and neutrons are in the center of the atom, making up the nucleus. • Electrons surround the nucleus. • Protons have a positive charge. • Electrons have a negative charge ...
Protons and neutrons are in the center of the atom making up the nucleus. Protons Neutrons and Electrons Worksheet W310 Everett Community College Tutoring Center Student Support Services Program Atomic symbol Atomic number Protons Neutrons Electrons Atomic mass Charge Pb 82 82 125 80 207 2 Se 34 34 45 34 79 0 Cr 24 24 28 21 52 3 F 9 9 10 9 19 0 Nb 41 41 52 35 93 5 P 15 15 16 18 31 -3.
In other words, why is there so much difference in behavior between two atoms when the only physical difference is one set of protons, electrons, and neutrons?
Protons neutrons and electrons how atoms differ worksheet answers Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Discuss the electronic and structural properties of an atom Key Takeaways Key Points An atom is composed of two regions: the nucleus, which is in the ...
Atomic Mass and Atomic Number Worksheet - Key. Name of. Element. Symbol. Atomic. Number. Atomic Mass. Protons. Neutrons. Electrons.1 page
Protons, Neutrons, and Electrons Practice Worksheet. Fill in the blanks in the following worksheet. Please keep in mind that the isotope represented by each space may NOT be the most common isotope or the one closest in atomic mass to the value on the periodic table.
34. $1.00. PDF. This simple worksheet shows students how to use the Periodic Table to find the element name, symbol, atomic number, mass number and then use that information to identify the number of protons, neutrons and electrons in the atom. Can be used as an introduction, guided practice or independent practice.
Protons, Neutrons and Electrons Homework. For Students 5th - 12th. Perfect for reviewing atomic components, this worksheet simply consists of a large, easy-to-read chart of 11 elements. Chemistry learners fill in the missing element symbol, number of protons, neutrons, and electrons, the atomic mass and...























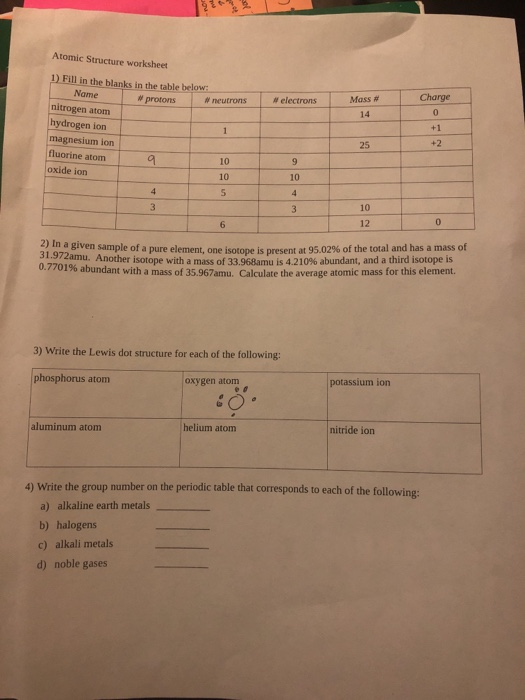
0 Response to "38 atoms protons neutrons electrons worksheet"
Post a Comment