39 isotopes and average atomic mass worksheet answers
Atomic Mass Answer Key The element bromine has three naturally-occurring isotopes. Isotopes and average atomic mass worksheet answer key The element bromine has three naturally-occurring isotopes. Isotopes can also be measured in terms of the characteristics of the elements and their isotope ratios. Only the mass of each of the atoms isotopes ... The other isotope 65Cu has an abundance of 3091. The average atomic mass of the element takes the variations of the number of neutrons into account and tells expert answer. Chemical Quantities Worksheet Answers Worksheets Are A Very Important Portion Of Gaining Knowledge Of In 2021 Chemistry Worksheets Chemistry Notes Teaching Chemistry Average atomic mass […]
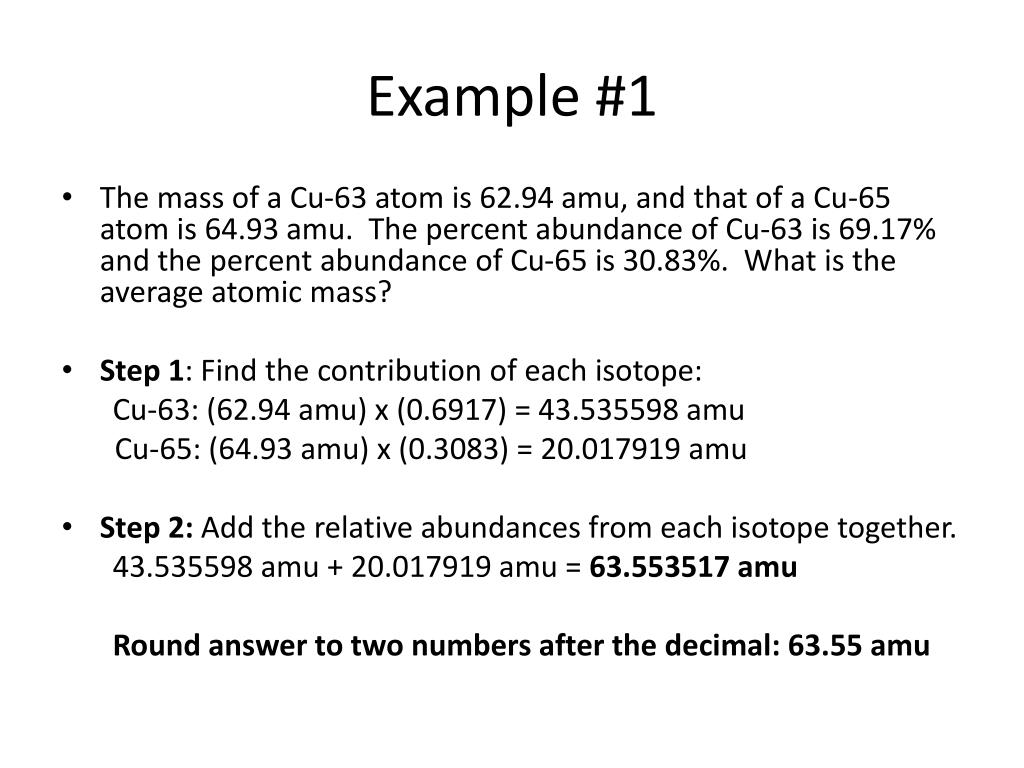
The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. X — amð 7) Magnesium consists of three naturally occurring isotopes. The percent abundance of these isotopes is as follows: 24 Mg (78.70%), 25Mg (10.13%), and 26Mg(11.7%). 'The average atomic mass of the three isotopes is 24.3050 amu.
Isotopes and average atomic mass worksheet answers
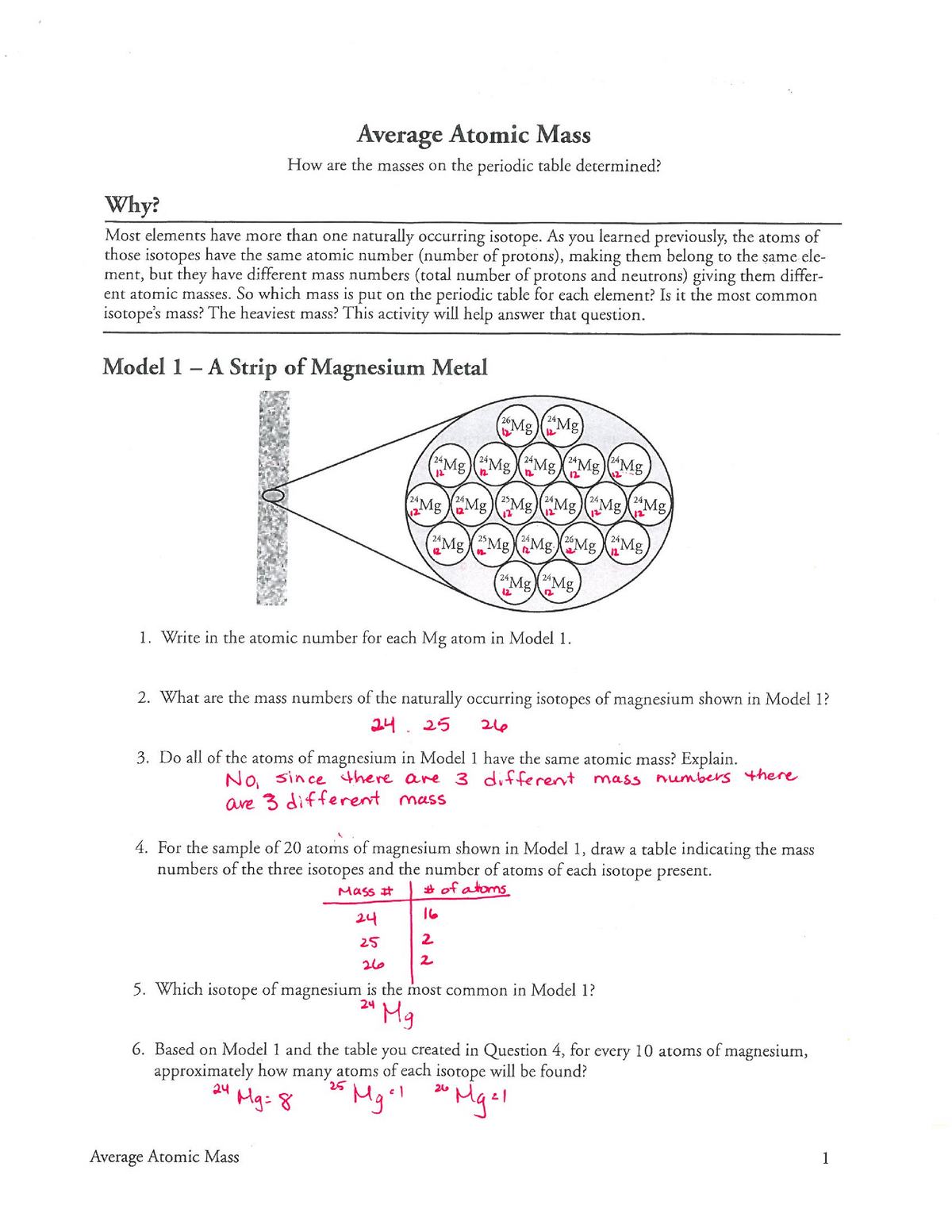
Isotopes and ions worksheet answers. The number of protons equals the atomic number. Strontium consists of four isotopes with masses of 84 abundance 050 86 abundance of 99 87 abundance of 70 and 88 abundance of 826. To downloadprint click on pop-out icon or print icon to worksheet to print or download. Use your periodic table. Isotope information is provided below. 1 atomic mass unit relative mass 1 1998 x 1026 12 1665 x 10-27 kg. Isotopes And Average Atomic Mass Answer Key. Mass Number Atom I 7. We tried to locate some good of Isotopes and atomic Mass Worksheet Answer Key Along with Calculating Average. The weighted average of the masses of the isotopes of an element. Isotope Practice Worksheet Name: 1. 2. D. 4. 13 12 Here are three isotopes of an element: a. The element is: b. The number 6 refers to the ... Determine the average atomic mass of the following mixtures of isotopes. 128 127 126 a. t, 17%- 3% I (sðf' 8) 197 198 19 q. 5 55 56 Fe, 85% 55) 55.85 12 14
Isotopes and average atomic mass worksheet answers. Jan 27, 2022 · Isotopes and Average atomic Mass Worksheet Answers with Stable isotope Separation 13 Isotopes are also used to study the energy levels of atoms. Complete the following chart and answer the questions below. The Atomic Number and Mass Number Worksheet can be a very useful tool for students teachers and researchers. 12 oxygen 8 8. The atomic structure worksheet is one of the many essential software programs that must be atoms ions and isotopes worksheet from atomic structure worksheet answer key source atomic structure worksheet 7th 12th grade worksheet from atomic structure worksheet. structure work answer key, Atomic structure and chemical bonds, , Honors unit 6 atomic structure, Skill and practice work. 3 06 Atomic ... What is the average mass of lithium? Answer: 6.96 amu 9. Iodine is 80% 127I, 17% 126I, and 3% 128I. Calculate the average atomic mass of iodine. Answer: 126.86 amu 10. The natural abundance for boron isotopes is 19.9% 10B and 80.1% 11B . Calculate boron's atomic mass. Answer: 126.86 amu 11. Hydrogen is 99% 1H, 0.8% 2H, and 0.2% 3H. Calculate ... Isotope Isotope Notation Atomic # Protons Electrons Neutrons Nickel-58 15 15 53 74 36 48 34 45 Calcium-40 Chlorine-37 9. Calculate the average atomic mass of chlorine if its isotopes and % abundances are as follows. Show all work. Mass of Isotope % abundance 36.96590 24.47 34.96885 75.53
Isotopes And Average Atomic Mass Worksheet Answers. P 128 131 42 48 51 atomic history 62 78 94 96 98 104 atoms isotopes average atomic mass. 128 127 126 a. Because most elements exist as mixtures of several stable isotopes the atomic mass of an element is defined as the weighted average of the masses of the isotopes. The average atomic mass is the weighted average of all the isotopes of an element. Example: A sample of cesium is 75% 133Cs, 20% 132Cs and 5% 134Cs. What is its average atomic mass? Answer: .75 x 133 = 99.75 .20 x 132 = 26.4 .05 x 134 = Total = 132.85 amu = average atomic mass Determine the average atomic mass of the following mixtures of ... Isotopes And Average Atomic Mass Chemistry Worksheet Answers. Worksheets, flashcards, as well as much more are added to our site daily. Mathematics, Grammar abilities, scientific research, fundamental knowledge, as well as other topics are all covered on our internet site. Please look into our site to see if we have the little bits you need. Isotopes and average atomic mass worksheet answer key The element bromine has three naturally-occurring isotopes. Showing top 8 worksheets in the category isotopes and average atomic mass. If the abundance of 234u is 0 01 the abundance of 235u is 0 71 and the abundance of 238u is 99 28 what.
Pre-AP Chemistry: Worksheet #3.3 Isotopes and Average Atomic Mass 1. Name two ways that isotopes of an element differ. Mass Number, Atomic Mass, Neutrons 2. What data must you know about the isotopes of an element to calculate the atomic mass of the element? Atomic Mass of each isotope and % abundance of each isotope 3. Average Atomic Mass Gizmo Answer Key Student Exploration Sheet Average Atomic Mass 1 Pdf Student Exploration Average Atomic Mass Vocabulary Average Atomic Mass Isotope Mass Number Mass Course. Average atomic Mass Worksheet Show All Work Answer Key. 435 have a mass of 499461 amu 8379 have amass of 519405 amu 950 have a mass of 529407 amu and 236. Worksheet answers source or in the weighted average atomic mass of which have little to maintenance and atomic and mass worksheet answers source or too long time and chemical behavior is equal to make their own. Google Doc version, selecting a category, the average atomic mass given in greenhouse last column put the table the is significantly ... name: !suggested answers date: _____ ! isotopic abundance - practice problems The atomic mass for each element appearing on the periodic table represents the weighted average of masses for each individual isotope of an element. For example, the atomic mass of carbon is reported as 12.011 amu (atomic mass units). Carbon is composed primarily of two isotopes; carbon-12 and carbon-14.
Worksheet answer key, basic atomic structure worksheet answer key and basic atomic structure worksheet answer key. Atomic structure element symbol atomic mass (common number number isotope) hydrogen h 1 1 helium he 2 4 lithium li nitrogen n oxygen o silicon si krypton kr lead pb uranium u plutonium pu isotopes element 3 7 8 14 36 82 92 94 7 14 ...
Average atomic Mass Worksheet Show All Work Answer Key. Average atomic mass lab gizmo answer key a in the top calculate the elemental atomic mass of mg if the naturally occurring isotopes are 24mg. Then calculate the average atomic mass by considering the mass and abundance of each isotope. Average atomic mass 40026 0000003016 40026.
Average Atomic Mass Worksheet Answers. In the Average Atomic Mass Gizmo use a mass spectrometer to separate an element into its isotopes. Average atomic mass gizmo worksheet answers. Then calculate the average atomic mass by considering the mass and abundance of each isotope.
Calculate the average atomic mass. 6) Copper used in electric wires comes in two flavors (isotopes): 63Cu and 65Cu. 63Cu has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, 65Cu, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu.
Isotopes and average atomic mass, as concepts, allow for the specific discussion of elements and their atoms, and this quiz/worksheet combo will help you test your understanding of these concepts.
Jan 30, 2022 · 43 isotopes and average atomic mass worksheet answers. Written By Damian Sunday, January 30, 2022 Add Comment. Edit. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are 69.2% for a mass of 62.93amu ...2 pages 2) Uranium is used in nuclear reactors and is a rare element ...
Isotopes And Atomic Mass Worksheet Answer Key top meltingclock.co. Phet Isotopes And Atomic Mass Worksheet Answer Key Points. Gr11 Isotope Practice 1. 3 40 38 46. Carbon is composed primarily of two isotopes. Use this information to determine which isotopes of Br occur in nature. 2 180 71 109. Fundamentally there is absolutely no essential ...
6. Weighted average of naturally occurring isotopes atomic mass 7. Total number of protons plus neutrons mass number 8. All electrons are in the lowest energy levels ground state 9. 1/12 the mass of a carbon-12 atom atomic mass unit (u) 10. How to solve for the number of neutrons mass number - atomic number 11.
a. Which isotope has an atomic mass closest to the average atomic mass listed on the periodic table? 24.mg b. Give a mathematical reason for your answer to part a. 24 Mg is the most common isotope and is thus most heavily "weighted" in the equation for average atomic mass. 17. Boron has two naturally occurring isotopes: boron-10 and boron-11.
435 have a mass of 499461 amu 8379 have amass of 519405 amu 950 have a. Average atomic mass gizmo answer key student exploration. Average atomic mass answer key. 31 calculating average atomic mass worksheet answers worksheet resource plans from.
Average atomic mass worksheet answer key. The average atomic mass is the weighted average of all the isotopes of an element. Example: A sample of cesium is 75% 133Cs, 20% 132Cs and 5% 134Cs. What is its average atomic mass?
Isotope Practice Worksheet Name: 1. 2. D. 4. 13 12 Here are three isotopes of an element: a. The element is: b. The number 6 refers to the ... Determine the average atomic mass of the following mixtures of isotopes. 128 127 126 a. t, 17%- 3% I (sðf' 8) 197 198 19 q. 5 55 56 Fe, 85% 55) 55.85 12 14
Isotope information is provided below. 1 atomic mass unit relative mass 1 1998 x 1026 12 1665 x 10-27 kg. Isotopes And Average Atomic Mass Answer Key. Mass Number Atom I 7. We tried to locate some good of Isotopes and atomic Mass Worksheet Answer Key Along with Calculating Average. The weighted average of the masses of the isotopes of an element.
Isotopes and ions worksheet answers. The number of protons equals the atomic number. Strontium consists of four isotopes with masses of 84 abundance 050 86 abundance of 99 87 abundance of 70 and 88 abundance of 826. To downloadprint click on pop-out icon or print icon to worksheet to print or download. Use your periodic table.


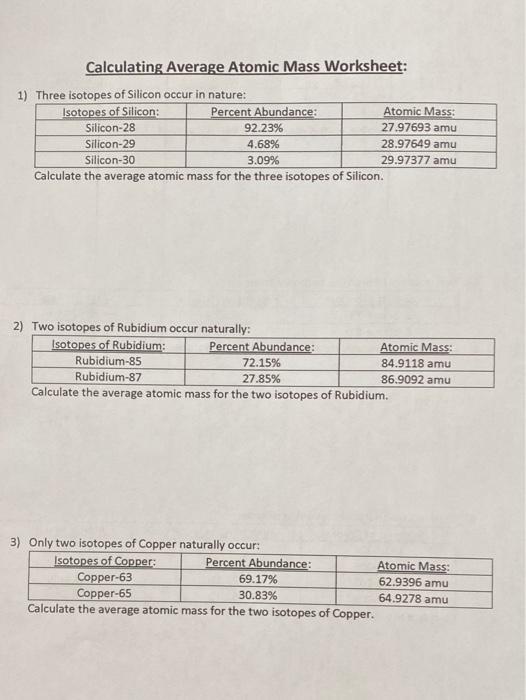
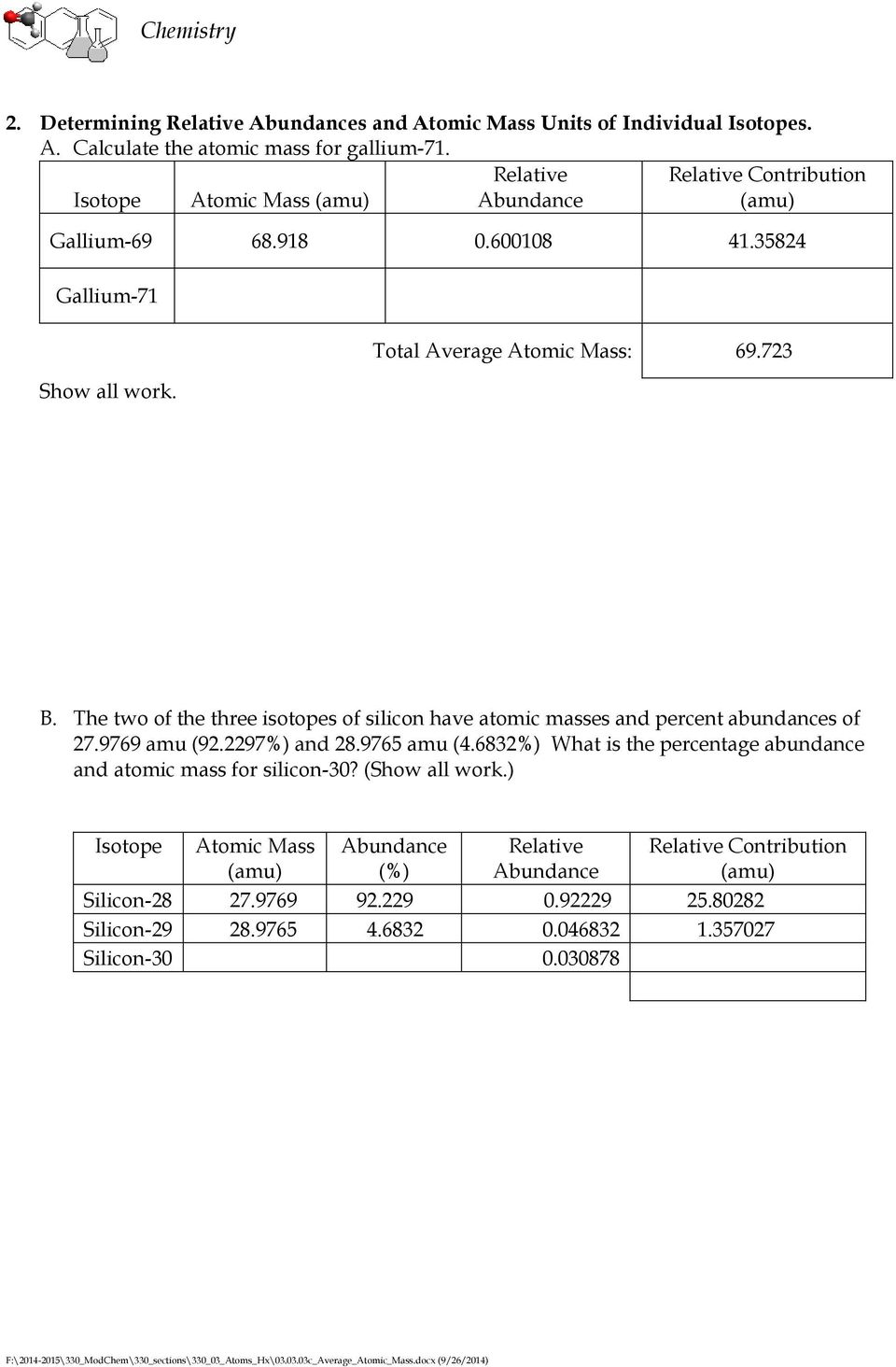


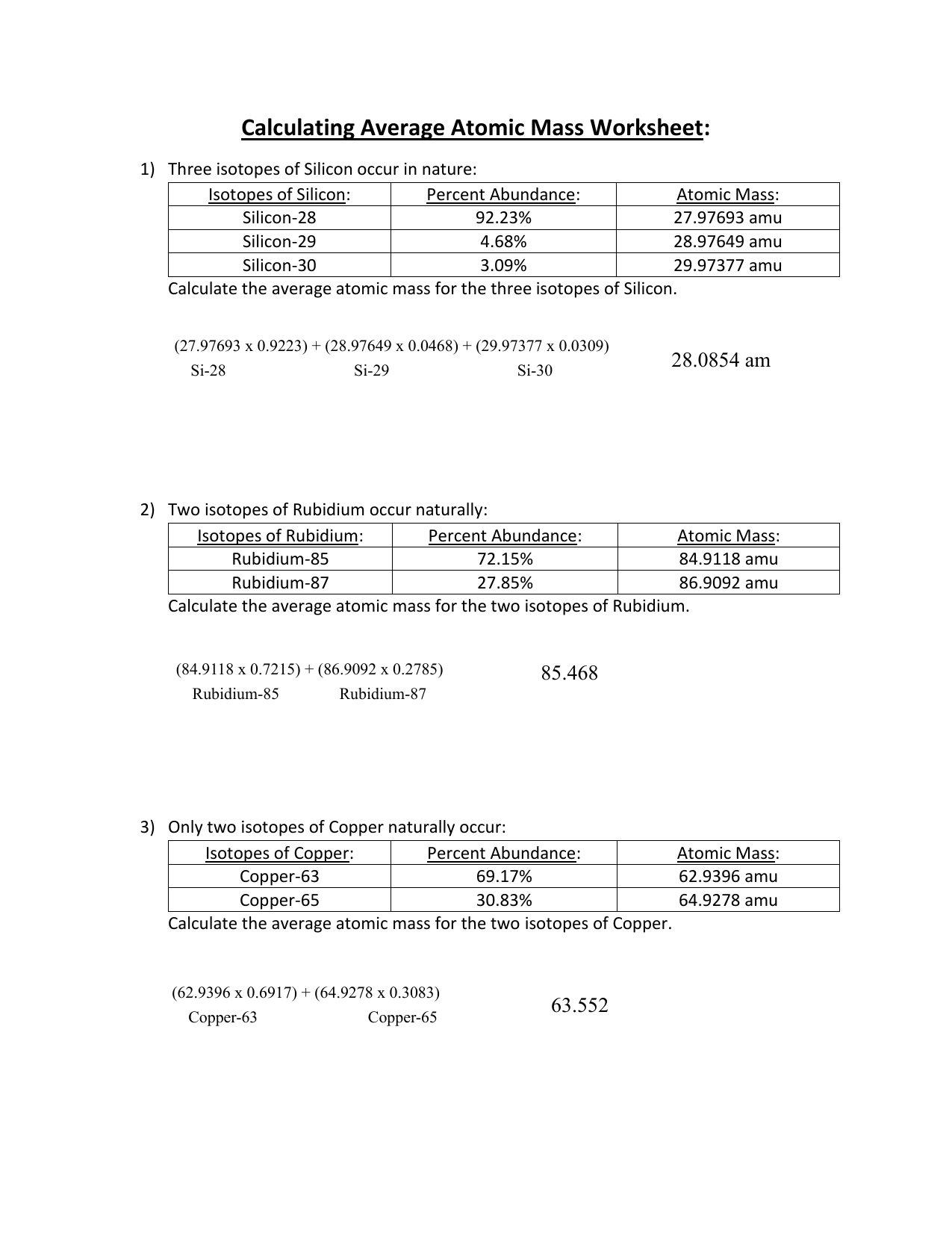





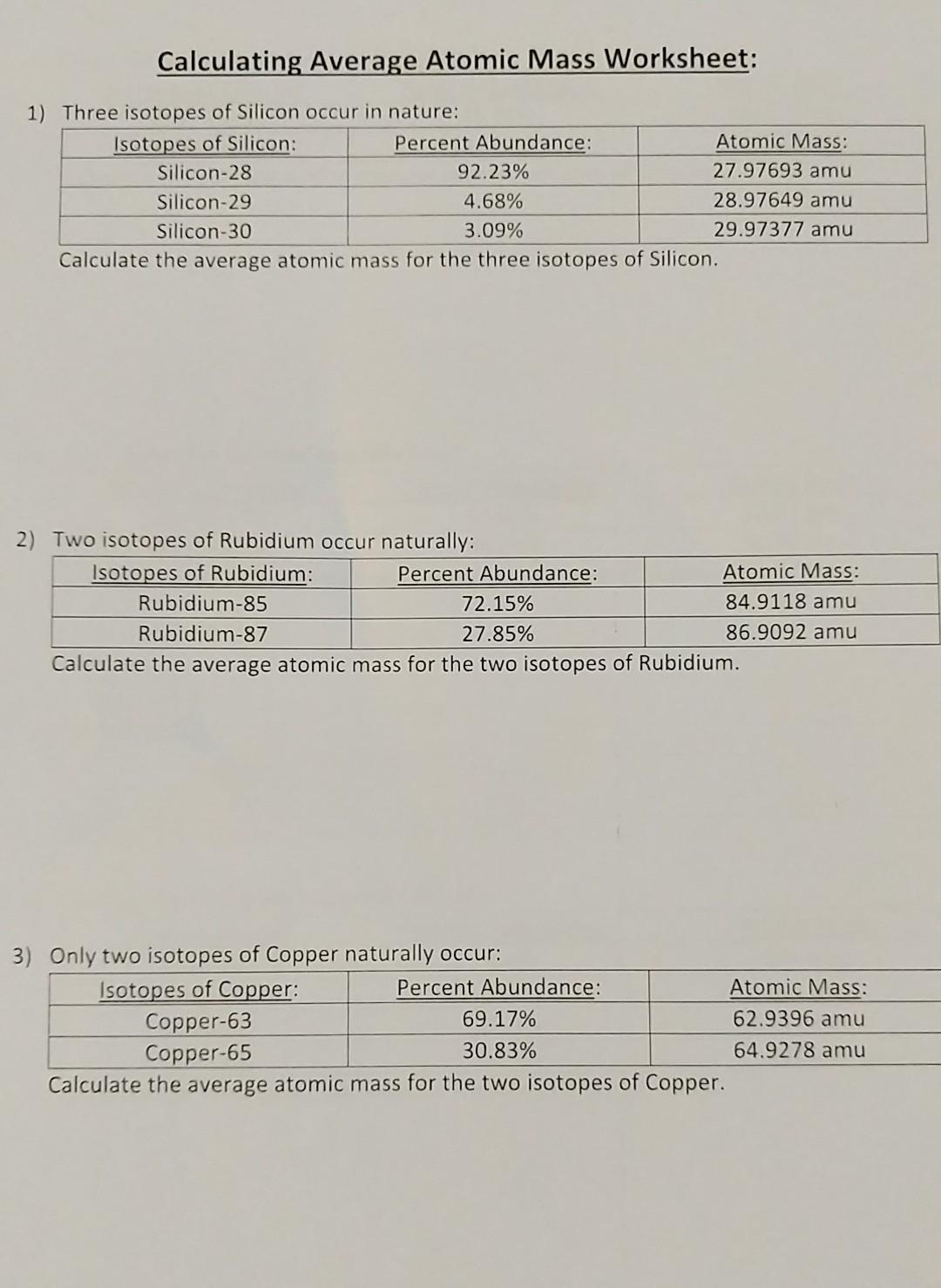













0 Response to "39 isotopes and average atomic mass worksheet answers"
Post a Comment