39 quantum numbers worksheet chemistry
PDF Worksheet - Quantum Numbers It consists of four numbers that act as coordinates to locate the electron's position. These numbers will refers only to the element's highest energy electron because the other fall into the same locations that have been described in the elements preceding it. 1. Principle Quantum Number (n): a. ENERGY LEVEL b. Indicate distance from the nucleus c. PDF HONORS WORKSHEET 7a: Quantum Numbers HONORS WORKSHEET 7a: Quantum Numbers 1. Write three possible sets of quantum numbers for the highest energy electrons in the aluminum atom. (3) n l m s Electron # 11 Electron # 12 Electron # 13 2. Which atomic theory is violated by the following sets of quantum numbers representing beryllium's outer shell electrons?
PDF Orbitals and Quantum Numbers Practice Questions Orbitals and Quantum Numbers Practice Questions 1. What are the shapes of s, p, and d orbitals respectively? s= spherical p = dumbbell d = cloverleaf 2. How many 1s orbitals are there in an atom? 4p orbitals? 4d orbitals? 1s: 1 4p: 3 4d: 5 3. What is the maximum number of orbitals with: n = 4 l = 1 3 (the 4p orbitals) n = 2 l = 2 none (l must ...

Quantum numbers worksheet chemistry
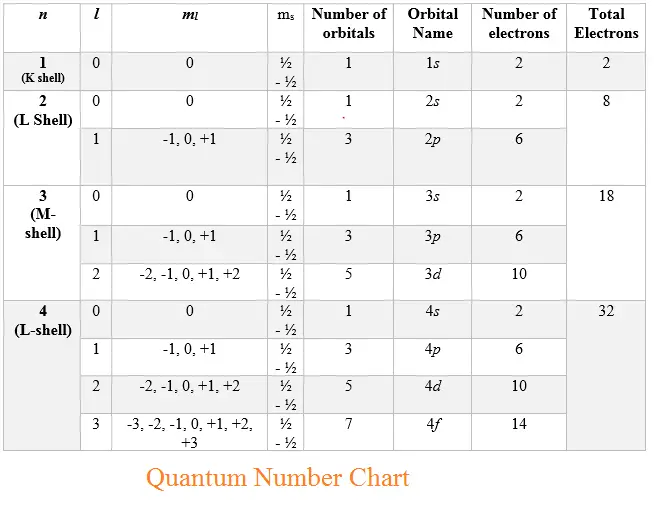
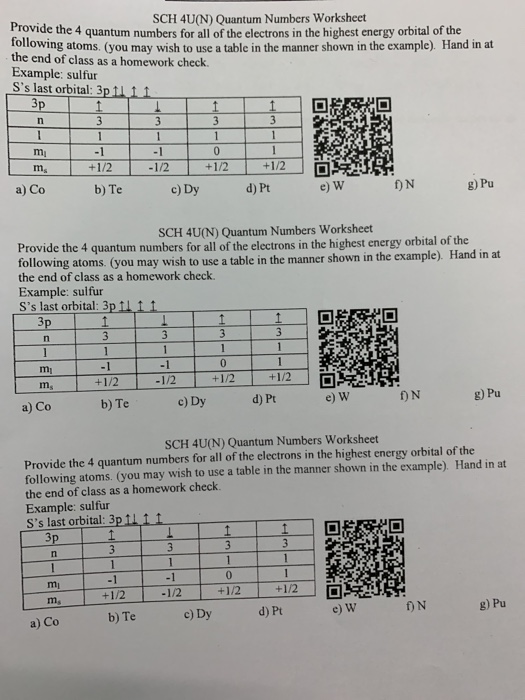
Lesson 2: Quantum Numbers - Grade 12 Chemistry (SCH4U) Pedagogy: this worksheet shows how much student have learned about Quantum Numbers after going over the lesson. Consolidation: this part is a game where students have 10 minutes to answer question that are related to quantum numbers. Lesson 1. Lesson 3. PDF Quantum Numbers and Electron Configurations • The principal quantum number (n) cannot be zero. The allowed values of n are therefore 1, 2, 3, 4, and so on. • The angular quantum number (l) can be any integer between 0 and n - 1. If n = 3, for example, l can be either 0, 1, or 2. • The magnetic quantum number (m) can be any integer between -l and +l. If l = 2, m can be either -2, -1, 0, +1, or +2. PDF Electron Configurations, Orbital Notation and Quantum Numbers QUANTUM NUMBERS AND ATOMIC ORBITALS Principal quantum number (n) 1, 2, 3, 4, 5, etc. Determines the total energy of the electron. Describes the energy level of the electron and refers to the average distance of the electron from the nucleus. 2n2 electrons may be assigned to an energy level. For n = 1, 2 electrons. For n = 2, 8 electrons, etc.
Quantum numbers worksheet chemistry. Quantum Numbers Lesson Plans & Worksheets Reviewed by Teachers In this chemistry worksheet, students complete 22 problems and fill in the blank questions on quantum numbers, electron configuration and hybridization of orbitals. + Lesson Planet: Curated OER The Modern Atom For Students 9th - 12th QUANTUM NUMBERS WORKSHEET 1. State the four ... QUANTUM NUMBERS WORKSHEET 1. State the four quantum numbers, then explain the possible values they may have and what they actually represent. n - Pricipal Quantum Number: represents the energy level the electron is in, linked to the periods of the periodic. Can be 1 to 7 l - Secondary Quantum Number/Orbital Shape Quantum number: represents the shape of Quantum Numbers Worksheet - worksheet Quantum numbers worksheet name 1. Write the 14 sets of quantum numbers that describe the 14 electrons of silicon si. N 3 l 0 b. Quantum numbers worksheet 1. N 3 l 1 c. These numbers will refers only to the element s highest energy electron because the other fall into the same locations that have been described in the elements preceding it. SP03 - Quantum Number and Energy Level - Worksheet ... Write the quantum numbers for the two electrons in a 3 s orbital. n = 3, l = 0, m = 0, m s = + ½ n = 3, l = 0, m = 0, m s = - ½ n = 3 , l = 0 , m l = 0 , m s = + ½ n = 3 , l = 0 , m l = 0 , m s = - ½ 7. The actual electron configuration of chromium is [Ar]4 s 1 3 d 5 . Do some research and explain why chromium has an anomalous electron arrangement.
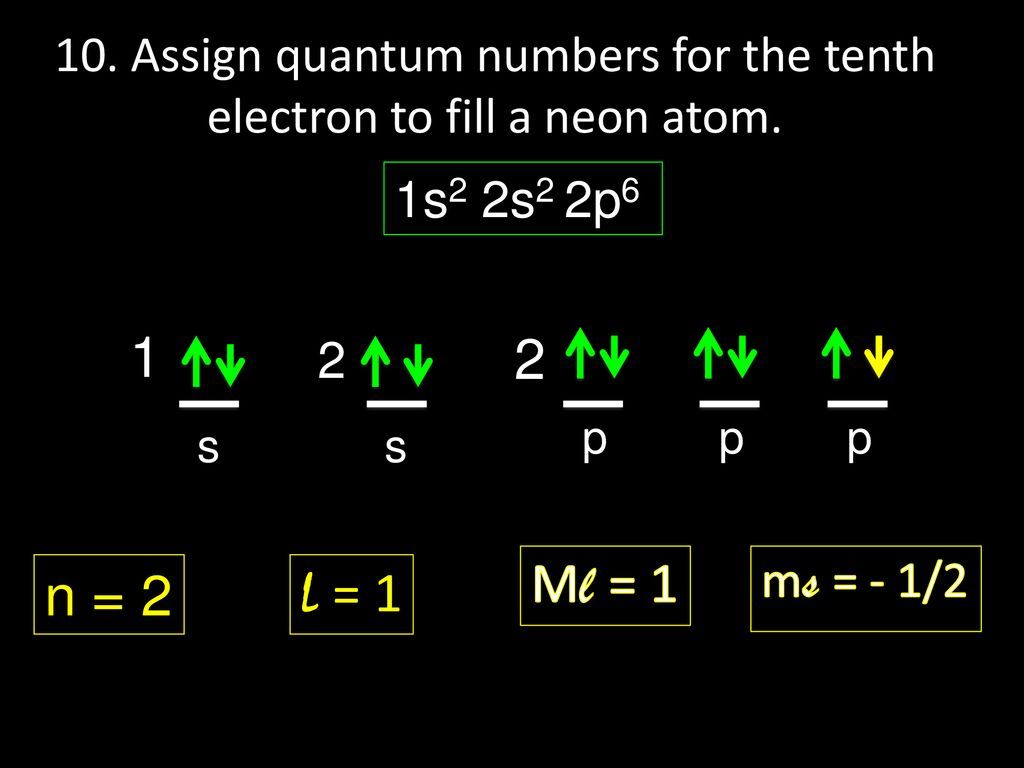
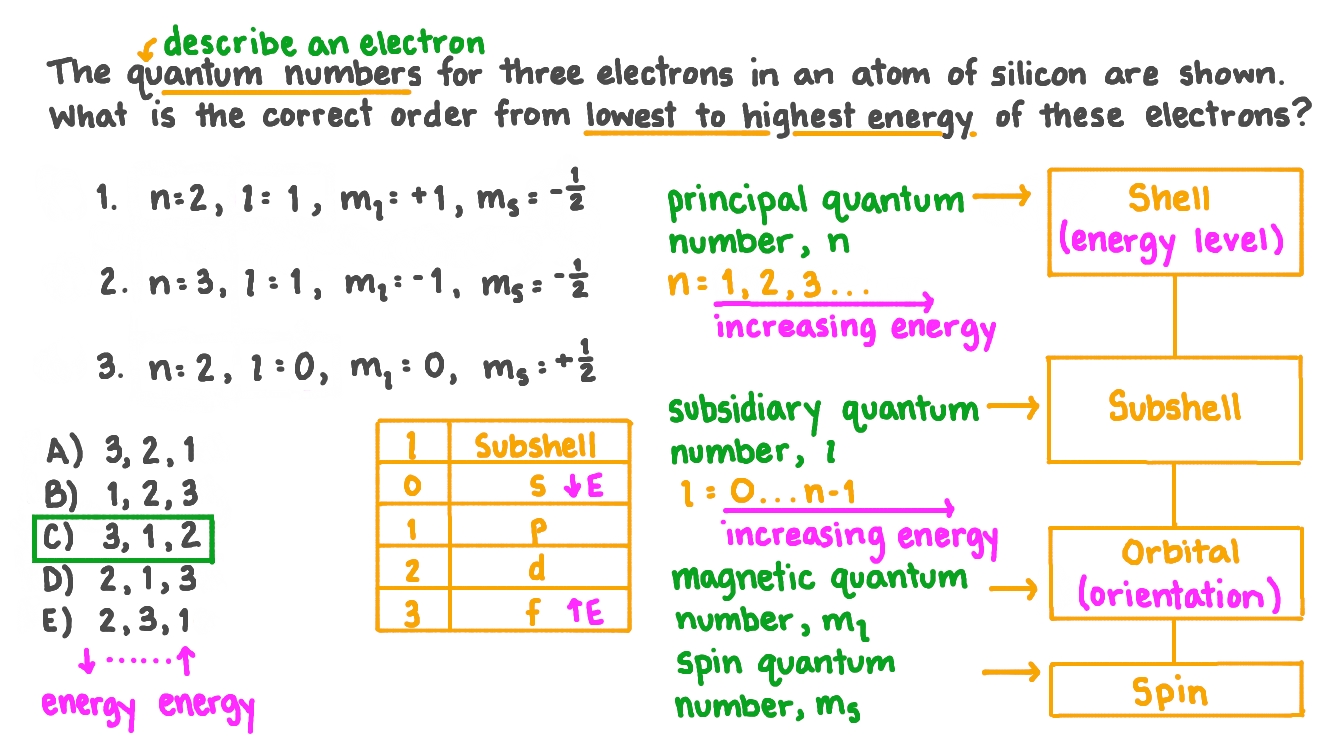
Intro To Electron Configuration Worksheet Answer Key ... Electron Configuration Practice Worksheet Last modified by. Electron Configurations - IntroductionThis lesson plan is a fantastic introduction to electron configurations. Electron Configuration 44 86 Chemistry Lessons Chemistry Classroom Electron Configuration Unit electron configurations quantum numbers - wksh 4 answers.Intro to electron configuration worksheet answer key. PDF CHEM1101 Worksheet 4: Quantum Chemistry Model 1: Light and ... Model 3: Atomic Orbitals and Quantum Numbers The wave functions for electrons in atoms are given the special name 'atomic orbitals'. As explored in Worksheet 3, the energy levels of hydrogen-like (one-electron)atoms are determined by a single quantum number, n. For other atoms, more quantities are ‐1 0 1 Lesson Worksheet:Quantum Numbers | Nagwa In this worksheet, we will practice using quantum numbers to describe an electron within an atom. Q1: The quantum numbers for three electrons in an atom of silicon are shown. What is the correct order from lowest to highest energy of these electrons? 𝑛 = 2, 𝑙 = 1, 𝑚 = + 1, 𝑚 = − 1 2 . 𝑛 = 3, 𝑙 = 1, 𝑚 = − 1, 𝑚 = − 1 ... Quantum Numbers (Principal, Azimuthal, Magnetic and Spin ... Four quantum numbers can be used to completely describe all the attributes of a given electron belonging to an atom, these are: Principal quantum number, denoted by n. Orbital angular momentum quantum number (or azimuthal quantum number), denoted by l. Magnetic quantum number, denoted by m . The electron spin quantum number, denoted by m s.
Quantum Numbers Worksheets & Teaching Resources | TpT Quantum numbers is a subtopic of advanced chemistry that can often confuse students. This elegant worksheet helps students see the definite pattern to quantum numbers. Once they discover the pattern, the "mind-blowing effects" of quantum numbers rapidly fades away. Beautifully illustrated and elegan DOC QUANTUM NUMBERS WORKSHEET - Lakeside High School QUANTUM NUMBERS WORKSHEET Name ________________________________ 1. State the four quantum numbers and the possible values they may have. 2. Name the orbitals described by the following quantum numbers a. n = 3, L = 0 b. n = 3, L = 1 c. n = 3, L = 2 d. n = 5, L = 0 3. Give the n and L values for the following orbitals DOC Worksheet - Quantum Numbers - Mrs. Wentzel's Chemistry ... Quantum Numbers Practice Problems. Fill in the orbital notation (arrows) below, then write the four quantum numbers which describe the location of the highest energy (last) electron of the following elements: Element 1s 2s 2p 3s 3p 4s 3d Quantum Numbers 1. Al 2. CBSE Class 11 Orbitals and Quantum Numbers Worksheet A Download printable Chemistry Class 11 Worksheets in pdf format, CBSE Class 11 Orbitals and Quantum Numbers Worksheet A has been prepared as per the latest syllabus and exam pattern issued by CBSE, NCERT and KVS. Also download free pdf Chemistry Class 11 Assignments and practice them daily to get better marks in tests and exams for Grade 11. Free chapter wise worksheets with answers have been ...
DOCX QUANTUM NUMBERS WORKSHEET - Hudson City School District QUANTUM NUMBERS WORKSHEETName ________________________________. 1. State the four quantum numbers and the possible values they may have. 2. Name the orbitals described by the following quantum number. a. n = 3, l = 0 b. n = 3, l = 1 c. n = 3, l = 2 d. n = 5, = 0. 3.
QUANTUM NUMBERS WORKSHEET - justonly b. The subshell with the quantum numbers n=4, l=2 is ______. c. The ml values for a d orbital are .5 pages
Worksheet – Quantum Numbers Quantum Numbers Notes and Practice. This is our final way to describe the location of an electron. It consists of four numbers that act as coordinates to.2 pages
Quantum Numbers Worksheet Answer Key - worksheet Quantum numbers worksheet 1. Answer the following questions. A the quantum number n describes the of an atomic orbital. Quantum numbers worksheet key 1. First primary quantum number n size of electron cloud n 1 up to in reality n 1 7second azimuthal or angular momentum quantum number l shape of electron cloud l 0 up to n 1.
Quantum Numbers (Worksheet) - Chemistry LibreTexts Worksheets: General Chemistry Worksheets: General Chemistry (Traditional) Quantum Numbers (Worksheet) Expand/collapse global location Quantum Numbers (Worksheet) ... Which of the following are permissible sets of quantum numbers for an electron in a hydrogen atom. For those combinations that are permissible, write the appropriate designation ...
PDF QUANTUM NUMBERS WORKSHEET - lee.k12.nc.us QUANTUM NUMBERS WORKSHEET Name _____ 1. State the four quantum numbers and the possible values they may have. 2. Name the orbitals described by the following quantum numbers a. n = 3, L = 0 b. n = 3, L = 1 c. n = 3, L = 2 d. n = 5, L = 0 3. Give the n and L values for the following orbitals a. 1s b. 3s c. 2p d. 4d e. 5f
SCH4U0 - 1 - 2 - WORKSHEET - Quantum Numbers.docx - SCH4U0 ... View SCH4U0 - 1 - 2 - WORKSHEET - Quantum Numbers.docx from CHEMISTRY 4U at Mississauga Secondary School. SCH4U0 Quantum Numbers Name: _ Date: _ Quantum Numbers Worksheet 1. What are quantum
PDF Chem 115 POGIL Worksheet - Week #9 Quantum Mechanical ... quantum numbers, designated n, l, and ml. Each quantum number is associated with a particular aspect of the electron's behavior and the distribution of its probability in space around the nucleus of the atom. Principal quantum number, n Determines energy of the one-electron atom by the equation, Allowed values are n = 1, 2, 3, ...
Quantum Numbers (Worksheet) - Chemistry LibreTexts The LibreTexts libraries are Powered by MindTouch ® and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739.
PDF Quantum Numbers - Hudson City School District QUANTUM NUMBERS WORKSHEET Name 1. State the four quantum numbers and the possible values they may have. 2. Name the orbitals described by the following quantum number 3. Give the n and I values for the following orbitals a. Is d. 4d e. 5f 4. Circle all of the following orbital destinations that are theoretically pos .ble. b. Ip c. 5d d. 2d e 4f f 5g 6i 5.
PDF Quantum Numbers and Atomic Orbitals - Angelo State University Each electron in an atom is described by four different quantum numbers. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. 1. Principal Quantum Number (n): n = 1, 2, 3, …, 8.
Quantum Numbers Worksheets | Teachers Pay Teachers Quantum numbers is a subtopic of advanced chemistry that can often confuse students. This elegant worksheet helps students see the definite pattern to quantum numbers. Once they discover the pattern, the "mind-blowing effects" of quantum numbers rapidly fades away. Beautifully illustrated and elegan.
Quantum number worksheet ID: 1632222 Language: English School subject: Chemistry Grade/level: GRADE 11 Age: 18+ Main content: Quantum number Other contents: Add to my workbooks (1) Download file pdf Embed in my website or blog Add to Google Classroom
PDF Electron Configurations, Orbital Notation and Quantum Numbers QUANTUM NUMBERS AND ATOMIC ORBITALS Principal quantum number (n) 1, 2, 3, 4, 5, etc. Determines the total energy of the electron. Describes the energy level of the electron and refers to the average distance of the electron from the nucleus. 2n2 electrons may be assigned to an energy level. For n = 1, 2 electrons. For n = 2, 8 electrons, etc.
PDF Quantum Numbers and Electron Configurations • The principal quantum number (n) cannot be zero. The allowed values of n are therefore 1, 2, 3, 4, and so on. • The angular quantum number (l) can be any integer between 0 and n - 1. If n = 3, for example, l can be either 0, 1, or 2. • The magnetic quantum number (m) can be any integer between -l and +l. If l = 2, m can be either -2, -1, 0, +1, or +2.
Lesson 2: Quantum Numbers - Grade 12 Chemistry (SCH4U) Pedagogy: this worksheet shows how much student have learned about Quantum Numbers after going over the lesson. Consolidation: this part is a game where students have 10 minutes to answer question that are related to quantum numbers. Lesson 1. Lesson 3.



























0 Response to "39 quantum numbers worksheet chemistry"
Post a Comment