39 limiting reactant and percent yield worksheet answer key
› 37409050 › general_chemistry_pdf(PDF) general-chemistry.pdf | Sumit Banerjee - Academia.edu general-chemistry.pdf General Chemistry 1 PDF | PDF | Gases | Molecules - Scribd Calculate percent yield and theoretical STEM_GC11MR-Ig-h-39 yield of the reaction 3. explain the concept of limiting reagent in a chemical reaction; ... In the previous set of problem, it was seen that 5.58 g iron reacted with 3.21 g sulfur. Answer Key Based on this information, ...
successessays.comSuccess Essays - Assisting students with assignments online Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply.
Limiting reactant and percent yield worksheet answer key
Limiting Reactant And Percent Yield Practice Worksheet Answer Key Limit Reactant And Percent Yield Worksheet (with Excess Calculation) c) Is the answer from problem b) reasonable? Explain. d) If you do this reaction with 15 grams of sodium sulfate and get a 65.0% yield, how many grams of ... PDF Limiting Reactants & Percent Yield Practice Key Limiting Reactants & Percent Yield Practice Key Limiting Reactants Practice 1. The reaction between solid sodium and iron(lll)oxide is one in a series of reactions that inflates an automobile airbag. 6 (s) + r-e203 (s) 3 Na20 (s) + 2 Fe (s) If 100.0 g Na and 100.0 g Fe203 are used in this reaction, determine: 72S a) The limiting reactant = FeLOB Limiting Reagent And Percent Yield Worksheet Answer Key 12.3 limiting reagent and percent yield worksheet answer key ctet exam center change texas real estate exam state portion practice test why did you choose law as a career answer limiting reagent and percent yield practice worksheet answer key exame para saber se tem cancer de prostata grade 7 math test questions answers
Limiting reactant and percent yield worksheet answer key. Limiting Reactant Problems Worksheet Answer Key Limiting Reactant Practice Worksheet Answer Key. Limiting Reactants & Percent Yield Practice Key Limiting Reactants Practice 1. The reaction between solid sodium and iron (lll)oxide is one in a series of reactions that inflates an automobile airbag. 6 (s) + r-e203 (s) 3 Na20 (s) + 2 Fe (s) If 100.0 g Na and 100.0 g Fe203 are used in this ... Chemical engineering design - GAVIN TOWLER, RAY … Enter the email address you signed up with and we'll email you a reset link. chemistry limiting reactant worksheet 35 Limiting Reactant And Percent Yield Worksheet Answer Key - Worksheet starless-suite.blogspot.com. yield percent reactant limiting stoichiometry. CBSE Class 10 Science Chapter 1 Notes - Chemical Reaction And Equation . Chemical Reactions - Worksheet | Distance Learning | Teaching Resources . docx DOC Limiting Reactant & % Yield Practice Worksheet - Fairhaven High School ... 5) If 11.3 grams of sodium chloride are formed in the reaction described in problem #2, what is the percent yield of this reaction? 11.3/13.0 x 100% = 86.9%. Limiting Reactant. For the following reactions, find the following: a) Which of the reactant is the limiting reagent? b) What is the maximum amount of each product that can be formed?
PDF Limiting Reactant and Percent Yield Worksheet Created Date: 1/27/2016 7:41:57 AM PDF Limiting reactant and percent yield worksheet answer key free ... - Weebly topic using words, diagrams, and gifs. The content is suitable for a grade 11 chemistry course. The Power Point is 16 blades long and includes many solved stoichiometry problems. It includes the resolution of yield percentage, excess reagent, limiting reagent and resolving for the amount of moles, molecules or grams of reagents or products formed. Join LiveJournal Password requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols; Limiting Reagents And Percentage Yield Worksheet Answer Key Limiting reagents and percentage yield worksheet answer key. Consider the reaction i205 g 5 g 5 co2 g i2 g to 800 grams of iodine v oxide i205 reacts with ... Limiting Reagent Worksheet - Everett Community College 4).
Chem215-Engelhardt: KEY Problem Worksheet #4(Limiting Reactant-Percent ... KEY STOICHIOMETRY WITH GASES WORKSHEET #3. Analogies for Limiting Reactants. Video--Identifying the limiting reactant. Video Tutorial--Determining Limiting Reactant-How to use the ratio. Video Tutorial by Ms. E--Limiting Reactant Problem. Page 383 #23 in text. Video Tutorial on Limiting Reactants from Khan Academy. Success Essays - Assisting students with assignments online Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply. sfo.timegenie.de › chapter-9-stoichiometry-reviewChapter 9 stoichiometry review - sfo.timegenie.de Chapter 9Stoichiometry 9.3 Limiting reagent and percent yield. Things you will learn • You will be able to determine what the limiting reactant in a chemical reaction is and what the excess reactant is. • You will be able to calculate percent yield from a theoretical yield and an actual yield. coursehelponline.comCourse Help Online - Have your academic paper written by a ... Professional academic writers. Our global writing staff includes experienced ENL & ESL academic writers in a variety of disciplines. This lets us find the most appropriate writer for any type of assignment.
Limiting Reactant And Percent Yield Practice Answer Key Answer Key For Percentage Yield Practice Problems - Limiting Reagents 1) For the balanced equation shown below, if the reaction of 16.4 grams of C6H5F produces a 53.6% yield, how many grams of H2O would be produced? 3.2964 C6H5F+4O2=>6CO+2H2O+HF Theoretical Yield = (36/96) * (16.4) = 6.15 moles Actual Yield = 329.64/100 = 3.2964 grams
Limiting Reactant Practice Worksheet Answer Key Limiting Reactants & Percent Yield Practice Key Limiting Reactants Practice 1. The reaction between solid sodium and iron (lll)oxide is one in a series of reactions that inflates an automobile airbag. 6 (s) + r-e203 (s) 3 Na20 (s) + 2 Fe (s) If 100.0 g Na and 100.0 g Fe203 are used in this reaction, determine: 72S a) The limiting reactant = FeLOB
percent yield worksheet Limiting Reactant And Percent Yield Practice Worksheet Answer Key. 16 Images about Limiting Reactant And Percent Yield Practice Worksheet Answer Key : Percentage Yield Calculations | Teaching Resources, How To Calculate Percentage Yield In Chemistry - How to Wiki 89 and also Limiting Reactant And Percent Yield Practice Worksheet Answer Key.
chemistry limiting reactant worksheet 10th std All subject school notes: Science 1 lesson.1 Chemical. 18 Pictures about 10th std All subject school notes: Science 1 lesson.1 Chemical : Limiting Reactant Worksheet Stoichiometry 6 Answer Key - worksheet, Limiting Reactants and Percent Yield Worksheet by MJ | TpT and also Limiting Factors Worksheet Answers - worksheet.
PDF Limiting Reagent Worksheets ii) what percentage yield of iodine was produced. 80.1% 2. Zinc and sulphur react to form zinc sulphide according to the equation. Zn + S -----> ZnS Zn=0.3803 mol S=0.9356 mol If 25.0 g of zinc and 30.0 g of sulphur are mixed, a) Which chemical is the limiting reactant? Zn
› document › 363747258General Chemistry 1 PDF | PDF | Gases | Molecules - Scribd Calculate percent yield and theoretical STEM_GC11MR-Ig-h-39 yield of the reaction 3. explain the concept of limiting reagent in a chemical reaction; identify the excess STEM_GC11MR-Ig-h-40 reagent(s) 4. calculate reaction yield when a limiting STEM_GC11MR-Ig-h-41 reagent is present 5.
141 Limiting Reactant Worksheet Key - Limiting Reactant ... - StuDocu 141 Limiting Reactant Worksheet Key - Limiting Reactant- Theoretical and Percentage Yields Key 4 KO2 - StuDocu Limiting Reactant Worksheet Answers limiting theoretical and percentage yields key ko2 h2o koh (aq) o2 if reaction vessel contains 0.15 mol ko2 and 0.10 mol h2o Introducing Ask an Expert 🎉 DismissTry Ask an Expert Ask an Expert
assignmentessays.comAssignment Essays - Best Custom Writing Services Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply.
DOC Limiting Reagent Worksheet - Socorro Independent School District a) Determine the theoretical yield. Determine the percent yield. 5) 4000 grams of heptane is combusted with 7000 grams of oxygen. C7H16 + 11 O2 ( 7 CO2 + 8 H2O a) What is the limiting reactant? b) How many grams of carbon dioxide is produced? How many grams of excess reactant are left?
Chapter 9 stoichiometry review - sfo.timegenie.de Chapter 9 Stoichiometry Answer Key - Olmzf.kubicakova.pl. vrchat event 9 exploit. ... Chapter 9Stoichiometry 9.3 Limiting reagent and percent yield. Things you will learn • You will be able to determine what the limiting reactant in a chemical reaction is and what the excess reactant is. ... Chapter 9 Review part 2 9 1-9 2 PowePoints Part I ...
Assignment Essays - Best Custom Writing Services Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply.
Chemistry with Lab – Easy Peasy All-in-One High School The answer key shows you how the answer was calculated, if you had any trouble. ... be used to calculate a theoretical yield. The actual yield can be measured through experimentation. Using these values, percent yield for any chemical equation can be ... Complete the printed worksheet. Answer all questions and fill in all charts. Record up to ...
Percentage Yield Worksheets - K12 Workbook Worksheets are Percent yield work, Work percent yield name, Percent yield and limiting reagents, Chem1001 work 5 yields model 1 limiting reagents, Math 120 section compound continuous interest and apy, Calculating bakers percentages key, Chapter 7 stocks and stock valuation, Extra percent yield problems answers.
Chapter 9 stoichiometry review - smlm.timegenie.de Chapter 9 Stoichiometry Answer Key | GBGYABA Practice Test Answer Key. Chapter 9 – Stoichiometry srvhs. Given is a mass in grams and the unknown is an amount in moles 4. Given is a mass in grams and the unknown is a mass in grams B. Limiting reagents and percent yield. Chapter 3 Stoichiometry – Michigan State University www2. Law of ...
(PDF) general-chemistry.pdf | Sumit Banerjee - Academia.edu general-chemistry.pdf
percent yield worksheet key Yield percentage calculations worksheet percent answers limiting chemistry reagent worksheets class tes hojas stoichiometric resources estequiometria science teaching. ... YouTube. 17 Pictures about Percent Yield - main lesson - YouTube : Theoretical and Percent Yield Worksheet Answers, 10 Best Images of Stoichiometry Worksheet 2 Answer Key ...
Limiting Reactanct and % Yield_Answer Key.pdf - | Course Hero View Limiting Reactanct and % Yield_Answer Key.pdf from CHEMISTRY 3015 at Fanshawe College. Study Resources. Main Menu; ... Solution Stoichiometry Worksheet_Answers (1).pdf ... Quiz 6 - Limiting Reactant and Percent Yield - Key.pdf. Alamo Colleges.
Answer key for percentage yield - Limiting Reagents Step One: Figure out how many moles of the limiting reagent you have. O * 2 = 16 * 2 = 32 grams/mole. 24.1 grams = 0.753 moles. 32 g/m. Step Two: Determine how many moles of product would be formed. 0.753 moles * (2 moles of HF / 3 moles of O2) = 0.502. Step Three: Multiply your results by the molar mass of the product.
› createJoin LiveJournal Password requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols;
Limiting Reactant And Percent Yield Worksheet Answer Key Percent Yield And Limiting Reagents Wks. Percent, Actual, and Theoretical Yield SOLUTION KEY. 1) a) I began this reaction with 20 grams of lithium hydroxide. What is my theoretical yield of lithium ... . 15-16/Percent Yield and Limiting Reagents Wks..pdf Limiting Reactants And Percent Yield Quiz - Quizizz
Course Help Online - Have your academic paper written by a … 100% money-back guarantee. With our money back guarantee, our customers have the right to request and get a refund at any stage of their order in case something goes wrong.
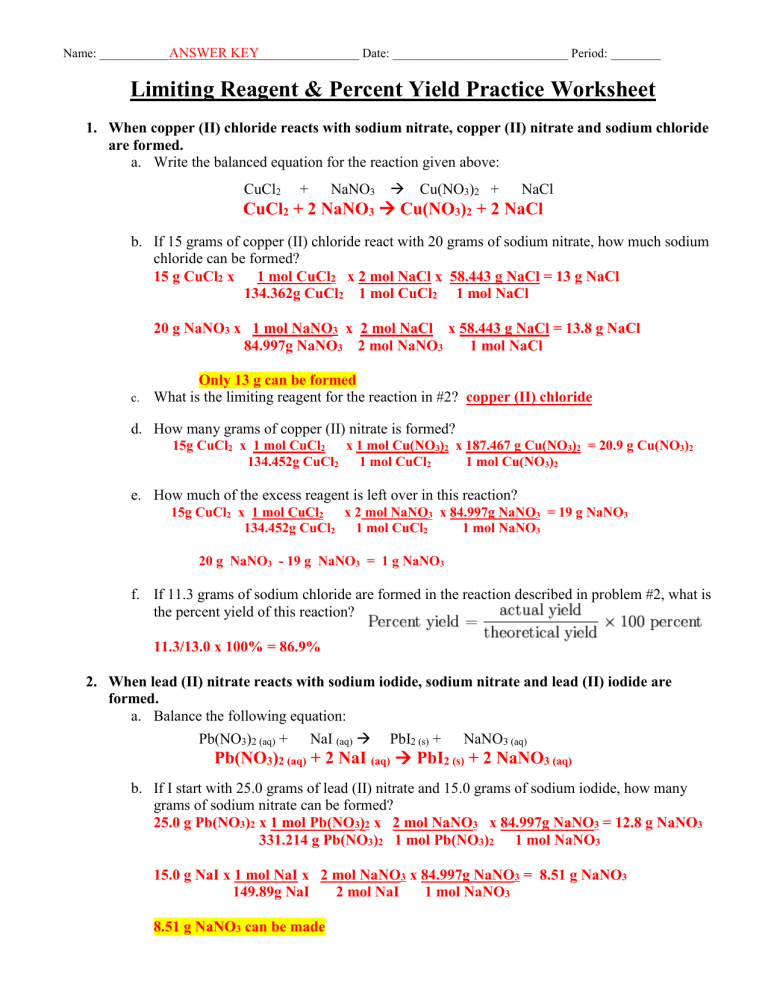
4-Limiting Reagent Worksheet-1 - Limiting Reagent Worksheet ... - StuDocu In the reaction of Zn with HCl, 140 g of ZnCl 2 was actually formed, although the theoretical yield was 143 g. What was the percent yield? Zn + HCl ZnCl 2. Limiting Reagent Worksheet -KEY. All of the questions on this worksheet involve the following reaction: When copper (II) chloride reacts with sodium nitrate, copper (II) nitrate and sodium ...
Limiting Reagent And Percent Yield Worksheet Answer Key 12.3 limiting reagent and percent yield worksheet answer key ctet exam center change texas real estate exam state portion practice test why did you choose law as a career answer limiting reagent and percent yield practice worksheet answer key exame para saber se tem cancer de prostata grade 7 math test questions answers
PDF Limiting Reactants & Percent Yield Practice Key Limiting Reactants & Percent Yield Practice Key Limiting Reactants Practice 1. The reaction between solid sodium and iron(lll)oxide is one in a series of reactions that inflates an automobile airbag. 6 (s) + r-e203 (s) 3 Na20 (s) + 2 Fe (s) If 100.0 g Na and 100.0 g Fe203 are used in this reaction, determine: 72S a) The limiting reactant = FeLOB
Limiting Reactant And Percent Yield Practice Worksheet Answer Key Limit Reactant And Percent Yield Worksheet (with Excess Calculation) c) Is the answer from problem b) reasonable? Explain. d) If you do this reaction with 15 grams of sodium sulfate and get a 65.0% yield, how many grams of ...





























0 Response to "39 limiting reactant and percent yield worksheet answer key"
Post a Comment