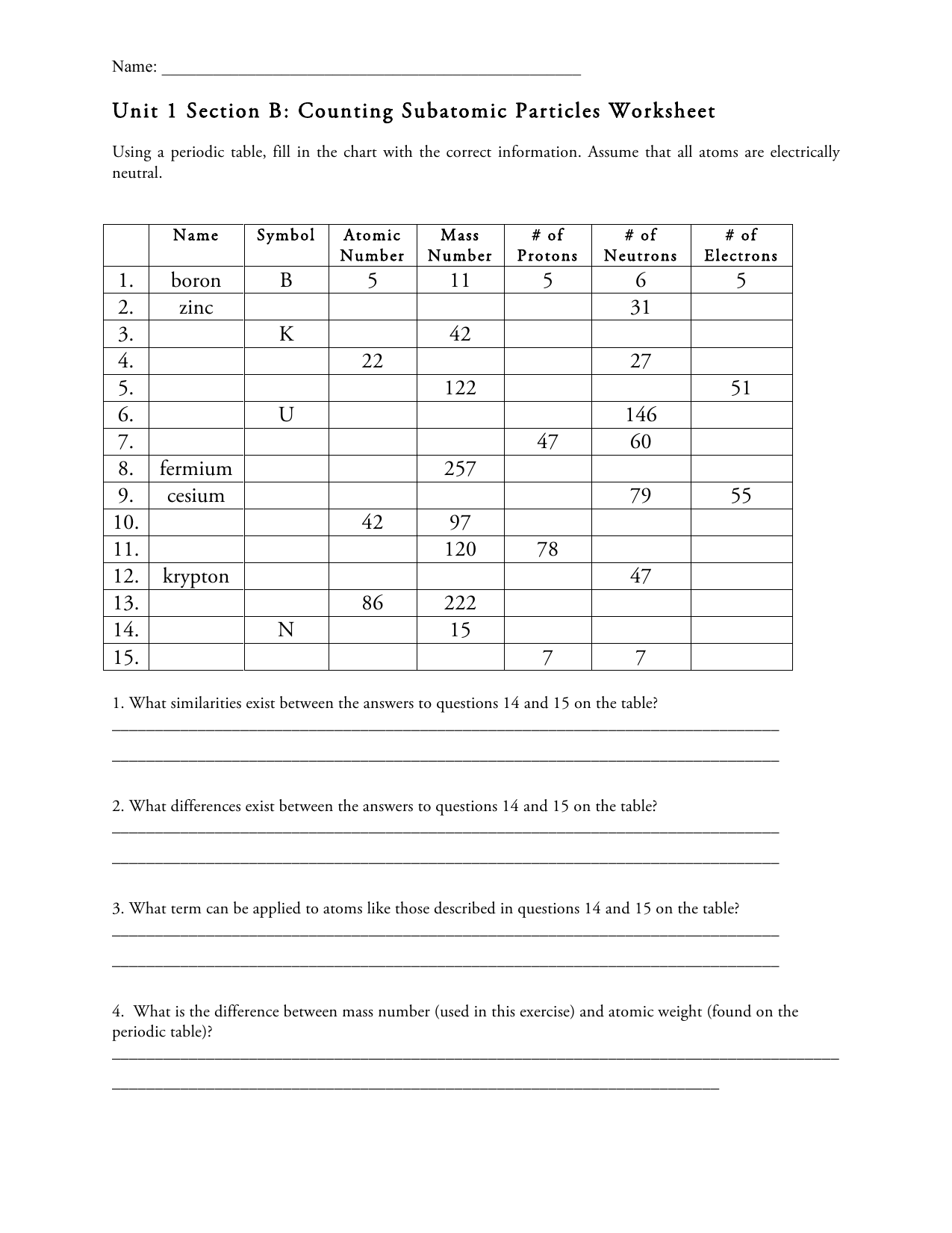
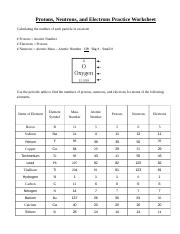
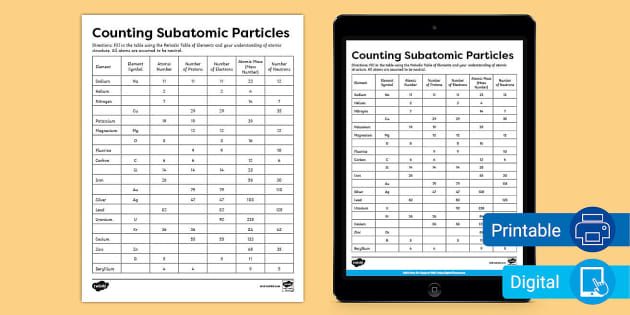
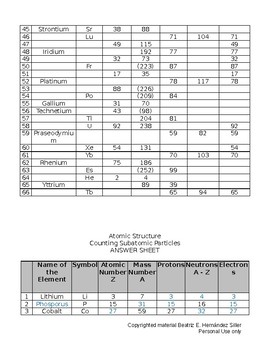
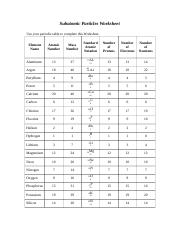
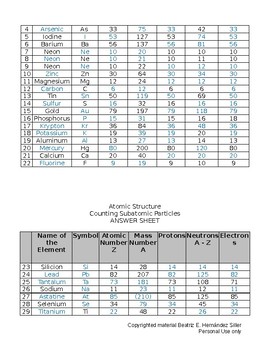
42 atoms and subatomic particles worksheet
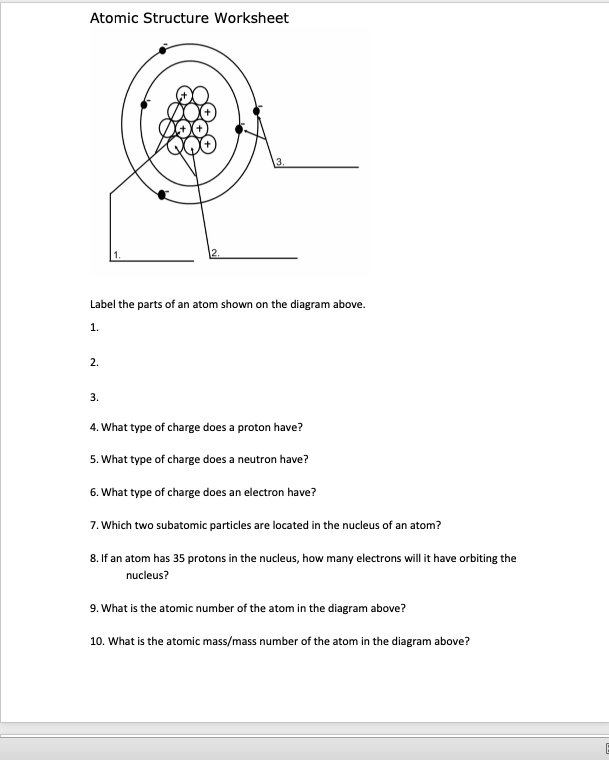
Basic Atomic Structure Worksheet Key - Neshaminy School District Basic Atomic Structure Worksheet and the 1. 2. 3. 4. 5. 6. 8. 9. The 3 particles of the atom are: Their respective charges are: The number of protons in one atom of ... Chemistry - Wikipedia Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds composed of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances.. In the scope of its subject, chemistry occupies an …
Atom Diagram - Universe Today They did not know it, but that was simply heated metals exchanging subatomic particles to become a new metal. Basic chemistry explains the atom best. It states that the fundamental building block ...
Atoms and subatomic particles worksheet
phet.colorado.edu › en › simulationBuild an Atom - Atoms | Atomic Structure | Isotope Symbols ... Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! electron | Definition, Mass, & Facts | Britannica 22.09.2022 · electron, lightest stable subatomic particle known. It carries a negative charge of 1.602176634 × 10−19 coulomb, which is considered the basic unit of electric charge. The rest mass of the electron is 9.1093837015 × 10−31 kg, which is only 11,836the mass of a proton. An electron is therefore considered nearly massless in comparison with a proton or a neutron, … letstalkscience.ca › educational-resourcesIntroduction to the Particle Theory of Matter | Let's Talk ... Dec 14, 2020 · Any particle smaller than an atom is called a subatomic particle. Protons, neutrons and electrons are all subatomic particles. Atoms of the same element are the same. Atoms of different elements are different. All of the atoms in carbon are the same. But carbon atoms are different from the atoms in nitrogen and oxygen.
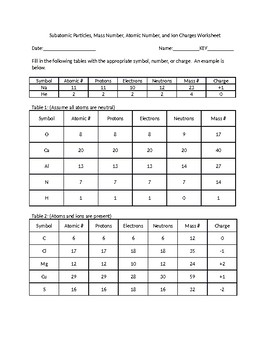
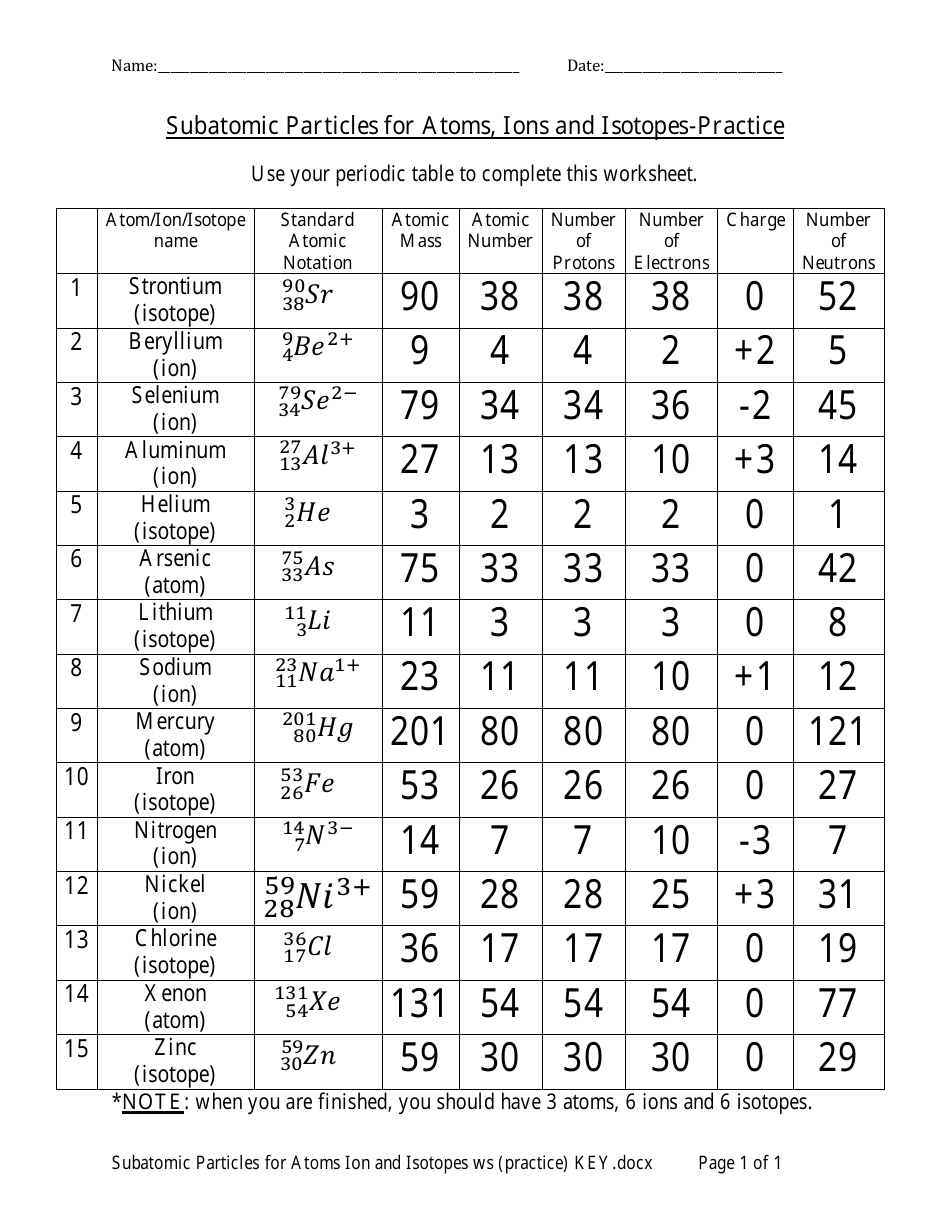
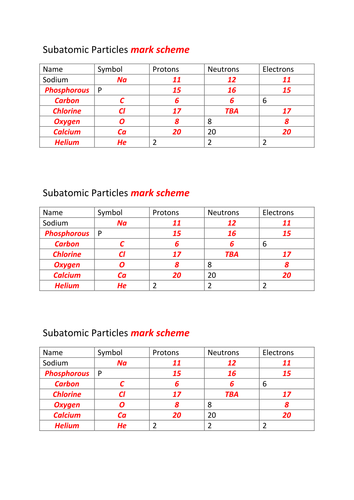
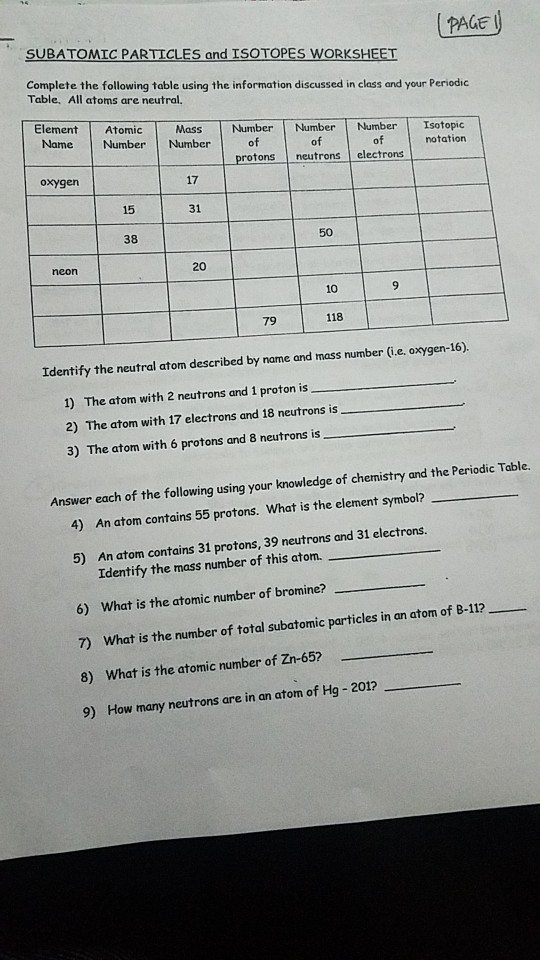
Atoms and subatomic particles worksheet. en.wikipedia.org › wiki › ChemistryChemistry - Wikipedia Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds composed of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. sciencespot.net › Pages › classchemThe Science Spot Version 1 includes atomic number, atomic mass, number of subatomic particles, Bohr diagram, and Lewis Structure. Version 2 is the one I use with my 8th grade students and includes all of the items in Version 1 as well as phase (solid, liquid, gas), melting/boiling points, discovery information, properties, and common uses. › science › physicalAtom Worksheets Here are the parts of an Atom: Electron; it is a subatomic particle with a negative electrical charge. The mass of an electron is so small that it is generally not even considered. Nucleus; the dense center of an atom containing neutrons and protons. Neutron; it is a subatomic particle within the nucleus of an atom that has a neutral charge. Loading... - BrainPop Loading... - BrainPop ... Loading...
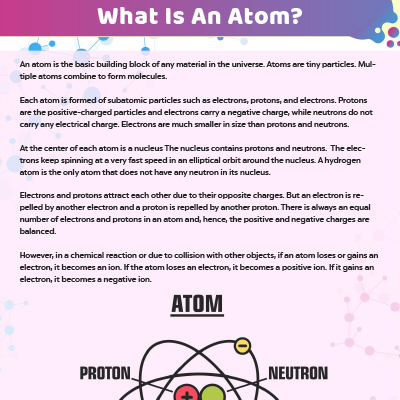
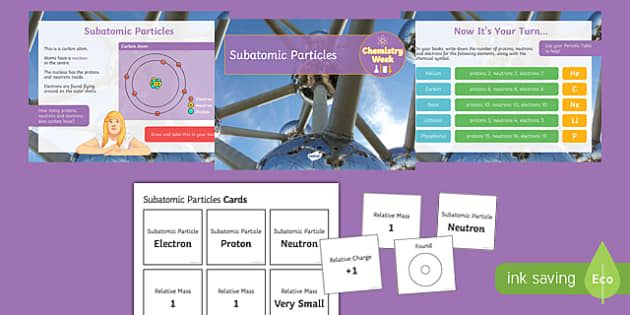
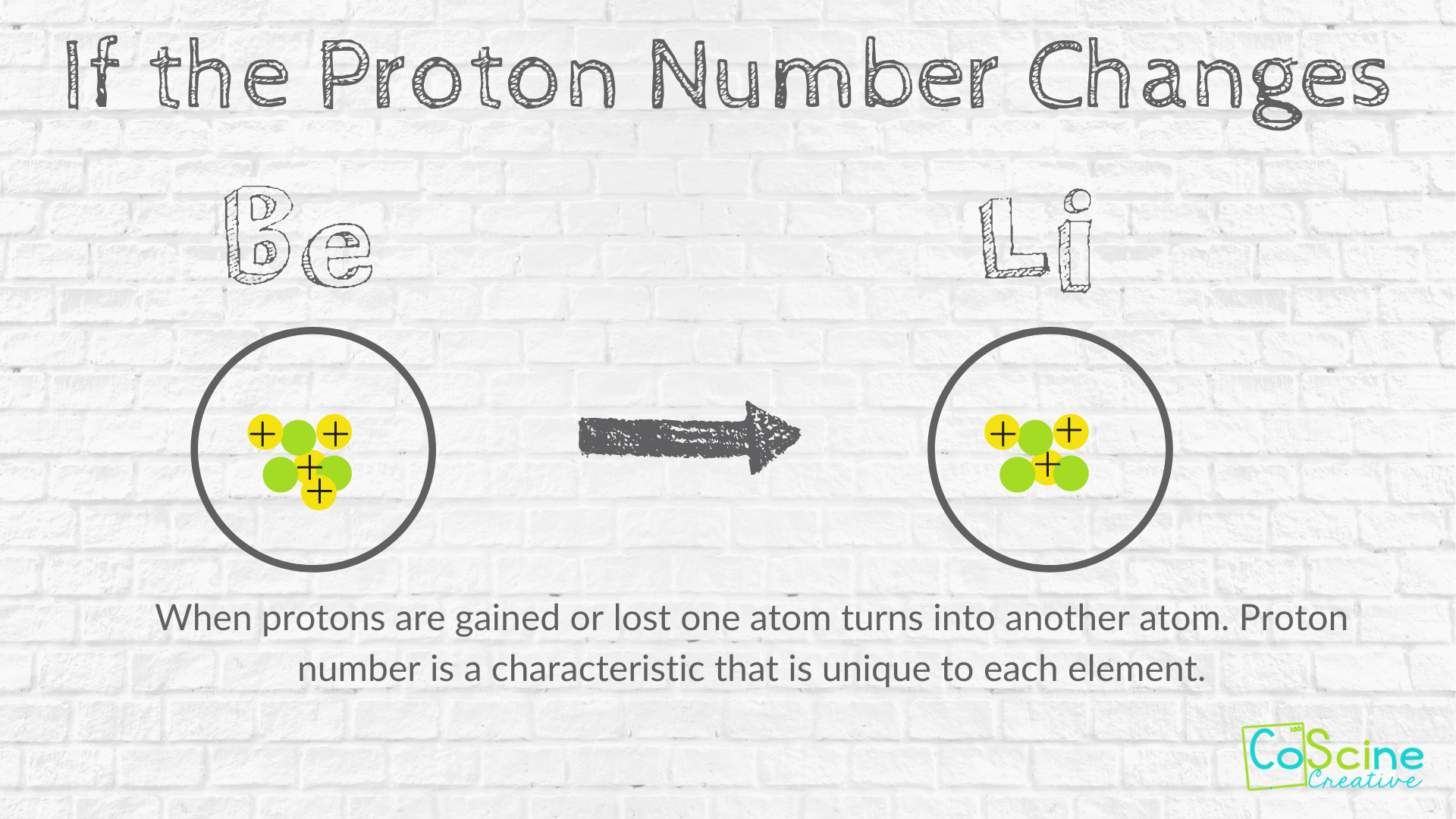
manoa.hawaii.edu › atoms-and-moleculesAtoms, Molecules, and Compounds | manoa.hawaii.edu ... Parts of Atoms. The particles that make up an atom are called subatomic particles (sub- means “smaller size”). These particles are the. proton (p +), which is positively (+) charged; electron (e –), which is negatively (–) charged; and; neutron (n 0), which has no charge, it is neutral (0). Protons and neutrons occupy the nucleus, or ... teaching.betterlesson.com › lesson › resourceNinth grade Lesson History of the Atom | BetterLesson So that I can start to gauge misconceptions that students hold such as the size of atoms, the size of the subatomic particles, how electrons move, and where the subatomic particles are located. This is an example of a student's modeling paper. Notice that his initial idea of an atom is more like a molecule with more than one atom together. letstalkscience.ca › educational-resourcesIntroduction to the Particle Theory of Matter | Let's Talk ... Dec 14, 2020 · Any particle smaller than an atom is called a subatomic particle. Protons, neutrons and electrons are all subatomic particles. Atoms of the same element are the same. Atoms of different elements are different. All of the atoms in carbon are the same. But carbon atoms are different from the atoms in nitrogen and oxygen. electron | Definition, Mass, & Facts | Britannica 22.09.2022 · electron, lightest stable subatomic particle known. It carries a negative charge of 1.602176634 × 10−19 coulomb, which is considered the basic unit of electric charge. The rest mass of the electron is 9.1093837015 × 10−31 kg, which is only 11,836the mass of a proton. An electron is therefore considered nearly massless in comparison with a proton or a neutron, …
phet.colorado.edu › en › simulationBuild an Atom - Atoms | Atomic Structure | Isotope Symbols ... Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!



















0 Response to "42 atoms and subatomic particles worksheet"
Post a Comment