42 average atomic mass worksheet
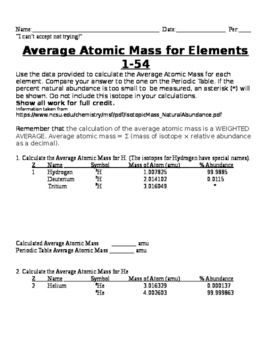
Isotopes and Atomic Mass - PhET Isotopes and Atomic Mass - PhET Atomic Structure Worksheet - Washoe County School District The atomic number gives the “identity “of an element as well as its location on the Periodic Table. No two different elements will have the atomic number. The of an element is the average mass of an element’s naturally occurring atoms, or isotopes, taking into account the of each isotope.
Lifestyle | Daily Life | News | The Sydney Morning Herald The latest Lifestyle | Daily Life news, tips, opinion and advice from The Sydney Morning Herald covering life and relationships, beauty, fashion, health & wellbeing
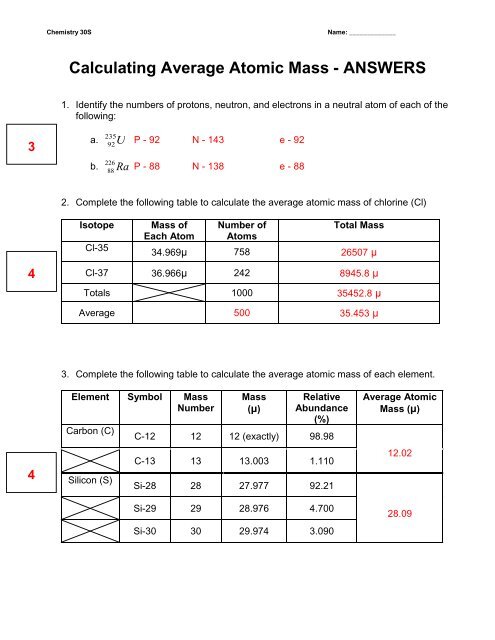
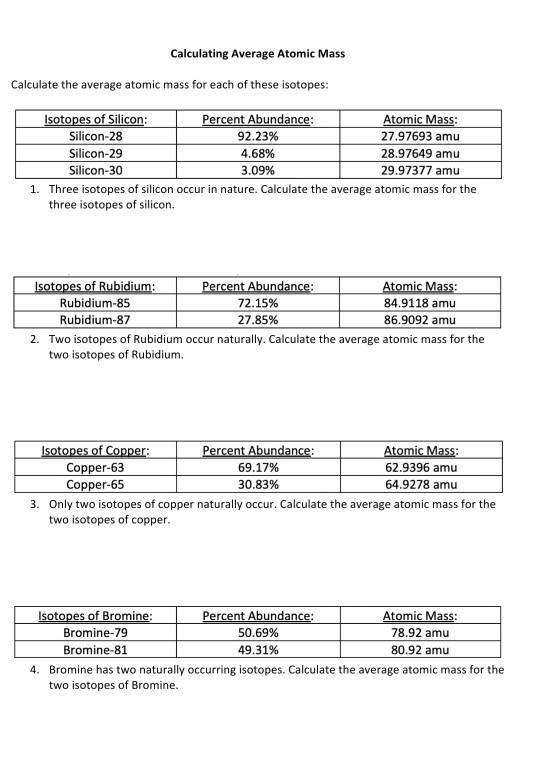
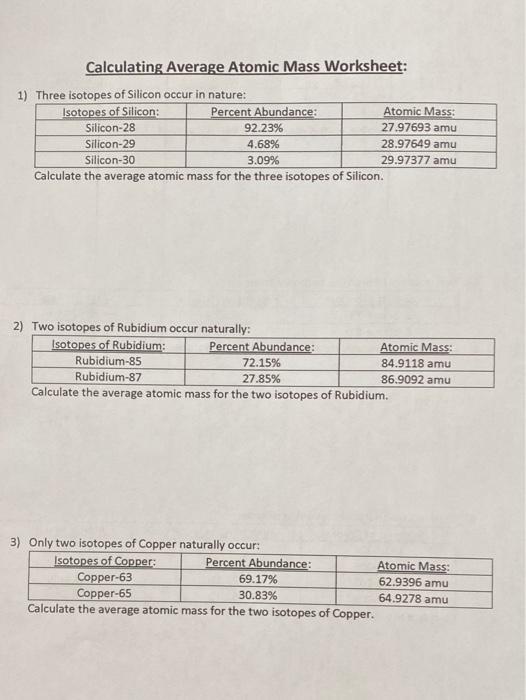
Average atomic mass worksheet
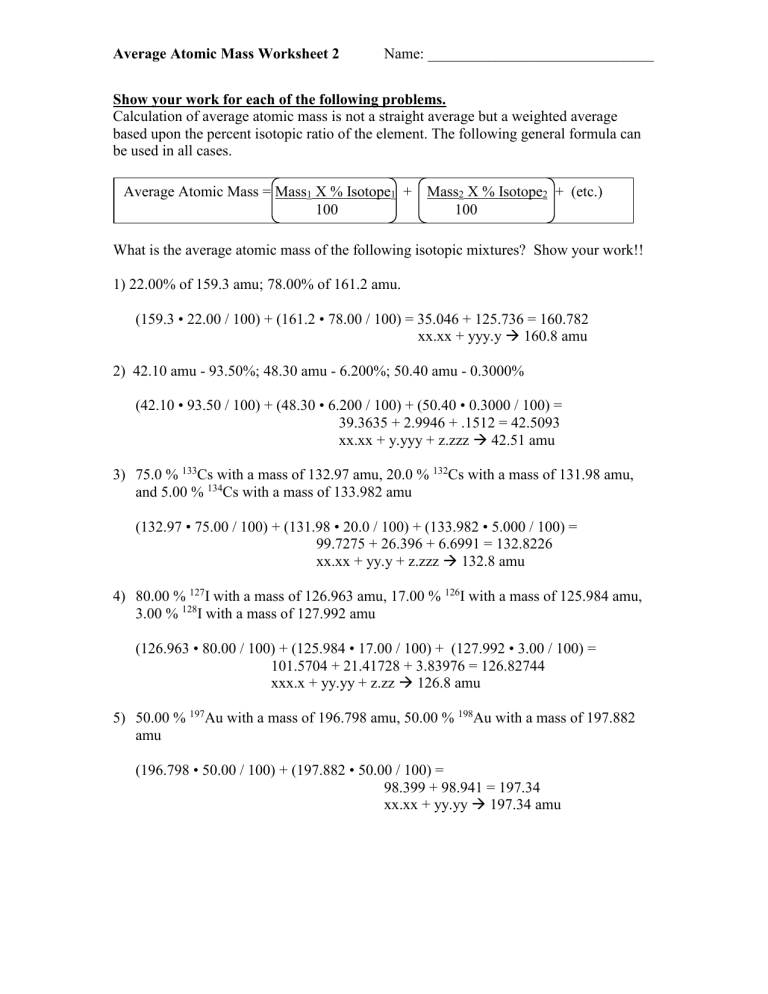
Quiz & Worksheet - Atomic Number and Mass Number | Study.com Print Atomic Number and Mass Number Worksheet 1. If Atom #1 has 19 protons and 22 neutrons, and Atom #2 has 20 protons and 22 neutrons, are these isotopes of the same element? HyperPhysics - GSU The hit rate reached about 50 million file hits per year in the fifth year and logging was suspended. Informal statistics from the server log indicate about 15 hits per user on average, so 50 million hits translates to over 3 million users per year. More recent probes have indicated about 2 million file accesses per day. Chemistry Lesson: Average Atomic Mass Calculations If silver is 51.84% Ag-107 with a mass of 106.9051 amu and the rest Ag-109 with a mass of 108.9048 amu, calculate silver’s atomic mass. Just like before, we need to take the abundance of Ag-107 times the mass of Ag-107 plus the abundance of Ag-109 times the mass of Ag-109.
Average atomic mass worksheet. 2.3: Calculating Atomic Masses (Problems) - Chemistry LibreTexts Average atomic masses listed by IUPAC are based on a study of experimental results. Bromine has two isotopes, 79 Br and 81 Br, whose masses (78.9183 and 80.9163 amu) and abundances (50.69% and 49.31%) were determined in earlier experiments. Calculate the average atomic mass of Br based on these experiments. Chemistry Lesson: Average Atomic Mass Calculations If silver is 51.84% Ag-107 with a mass of 106.9051 amu and the rest Ag-109 with a mass of 108.9048 amu, calculate silver’s atomic mass. Just like before, we need to take the abundance of Ag-107 times the mass of Ag-107 plus the abundance of Ag-109 times the mass of Ag-109. HyperPhysics - GSU The hit rate reached about 50 million file hits per year in the fifth year and logging was suspended. Informal statistics from the server log indicate about 15 hits per user on average, so 50 million hits translates to over 3 million users per year. More recent probes have indicated about 2 million file accesses per day. Quiz & Worksheet - Atomic Number and Mass Number | Study.com Print Atomic Number and Mass Number Worksheet 1. If Atom #1 has 19 protons and 22 neutrons, and Atom #2 has 20 protons and 22 neutrons, are these isotopes of the same element?



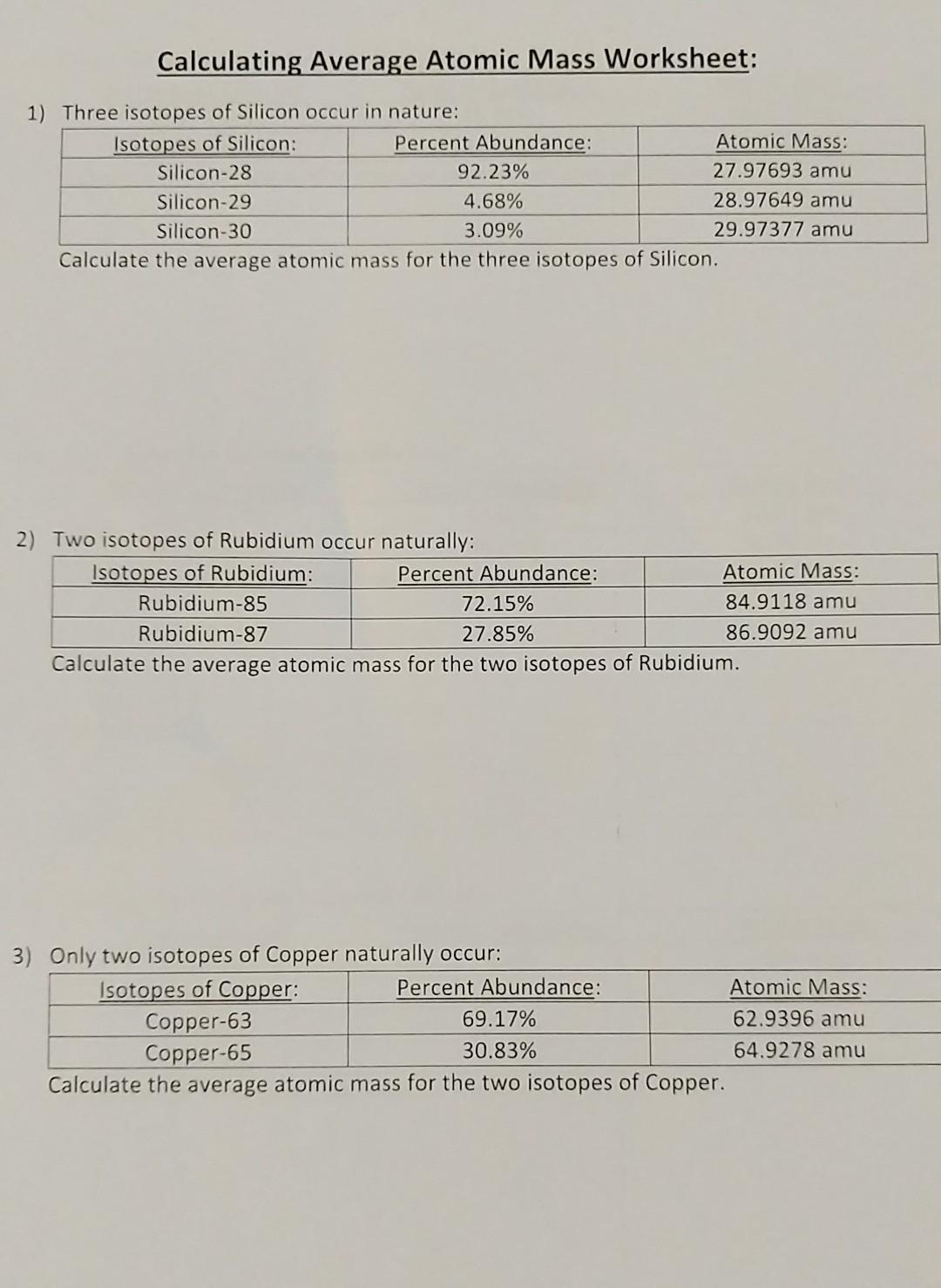

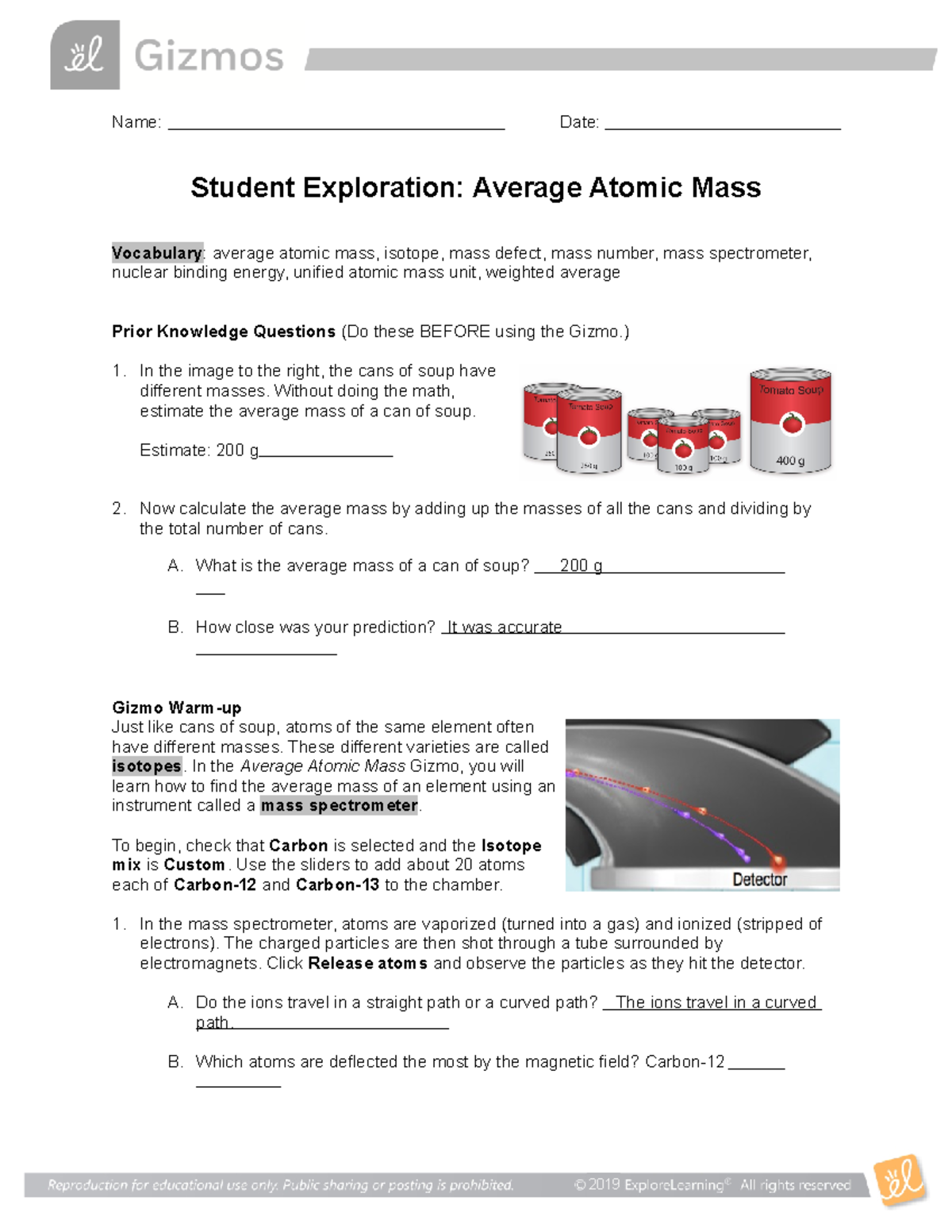


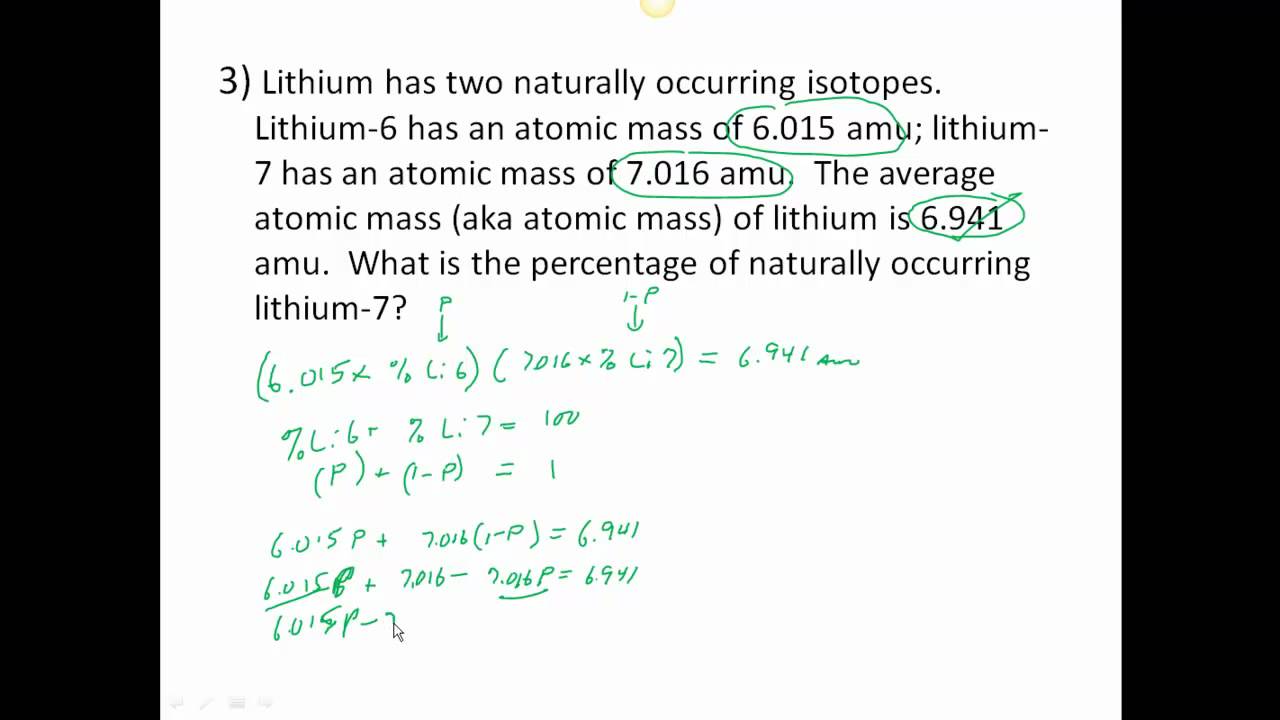















0 Response to "42 average atomic mass worksheet"
Post a Comment