43 calculating average atomic mass worksheet
Atomic Mass Worksheets Teaching Resources | Teachers Pay Teachers This worksheet walks students through the steps of calculating the average atomic mass of an element. Students will then practice through a couple of word problems. This worksheet includes an explanation of what average atomic mass is, an example of how to calculate amu, one conceptual question, and six practice problems. KEY INCLUDED. Calculating Average Atomic Mass Worksheet | Aurumscience.com. Calculating Average Atomic Mass, Part of understanding isotopes is realizing how their abundance determines the average atomic mass shown with each element of the periodic table. This worksheet will show students how these numbers are calculated, and help them understand why the atomic mass of oxygen is 15.99 AMU instead of simply 16 AMU.
Mass Calculations Worksheets - K12 Workbook Displaying all worksheets related to - Mass Calculations. Worksheets are Chm 4 pal atomic mass student name, Example exercise atomic mass and avogadros number, Mole calculations work, Mole calculation work, Mass volume and density review work, Work calculating empirical molecular formulas, Molar ratios and mass relationships in chemical equations, Chm 130 stoichiometry work.

Calculating average atomic mass worksheet
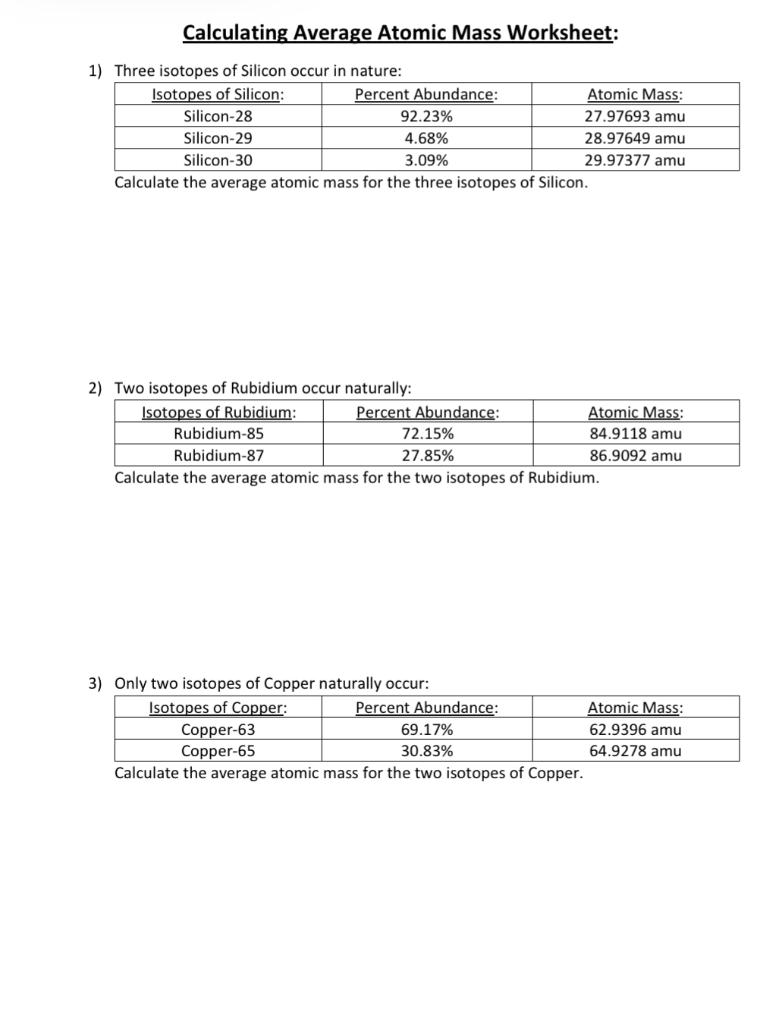
Chemistry: Average Atomic Mass Worksheet Flashcards | Quizlet Find the average atomic mass for Cl is 75.78% of Cl atoms are 35Cl with a mass of 34.96885271 amu and 24.22% are 37Cl with a mass of 36.96590260 amu. Find the average atomic mass for Mg if 78.99% of Mg atoms are 24Mg with a mass of 23.9850419 amu, 10.00% are 25Mg with a mass of 24.9858370 amu, and 11.01% are 26Mg with a mass of 25.9825930 amu. DOC Chemistry Worksheet - Forestville Central High School Calculate the average atomic mass of gold with the 50% being gold-197 and 50% being gold-198. Calculate the average atomic mass of lithium, which occurs as two isotopes that have the following atomic masses and abundances in nature: 6.017 u, 7.30% and 7.018 u, 92.70%. Hydrogen is 99% 1H, 0.8% 2H, and 0.2% 3H. Calculate its average atomic mass. PDF Calculating Average Atomic Mass Worksheet - SI PROGRAM Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Atomic Mass: 27.97693 amu 28.97649 amu 29.97377 amu Isoto es of Silicon: Silicon-28 Silicon-29 Silicon-30 Percent Abundance: 92.23% 4.68% 3.09% Calculate the average atomic mass for the three isotopes of Silicon. (-9 Y(.o ( zq.q 7377) 0/0 2) Two isotopes Of ...
Calculating average atomic mass worksheet. Calculating Average Atomic Mass Worksheet - Name ... - StuDocu The element Copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are: 69% for mass of 62 30% for mass of 64. Calculate the average atomic mass of Copper. Show ALL work for full credit.-----Calculate the average atomic mass of Sulfur if: 95% of all Sulfur atoms have a mass of 31 u, 0% ... Calculating density mass and volume worksheet formula for density is Density = mass / volume ( mass divided by volume ) The unit for density is grams per cubic centimeter (g/cm3) Calculate the densities of the following objects. You will need a calculator. Round all answers to the tenths place (1 place after the decimal) 7. A shoe box 8. a rock mass = 114.0 g volume = 538.5 cm3 mass = 22. Lesson Worksheet:Percentage Isotopic Abundance | Nagwa In this worksheet, we will practice calculating percentage isotopic abundances from the relative atomic mass and isotopic masses. Q1: Chlorine has two stable isotopes, 3 5 C l and 3 7 C l , with atomic masses 34.9689 u and 36.9659 u respectively. The relative abundance of 3 7 C l in an average sample of chlorine is 3 7 3 5 C l C l = 0. 3 1 9 6. Calculating Average Atomic Mass Chemistry Homework Worksheet Calculating Average Atomic Mass Chemistry Homework Worksheet, by, Science With Mrs Lau, 4.9, (21) $1.50, PDF, This chemistry homework page is perfect for students to practice calculating the average atomic mass using isotope data! The questions get progressively more involved as the page progresses.
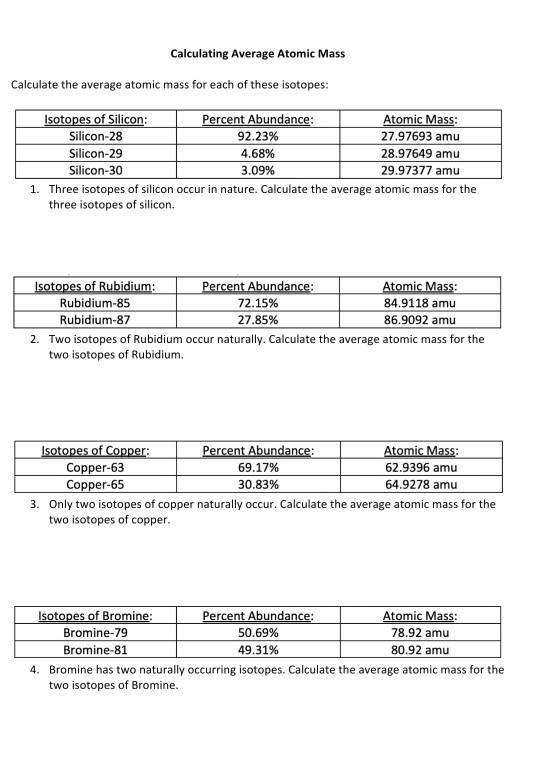
DOC Calculating Average Atomic Mass Worksheet Name______________________ 3. Calculate the average atomic mass of sulfur, if 95.00% of all sulfur atoms have a mass of 31.972 u, 0.76% , has a mass of 32.971u and 4.22% have a mass of 33.967u. 4. Naturally occurring strontium consists of four isotopes, Sr-84, Sr-86, Sr-87 and Sr-88. Below is the data concerning strontium: Sr-84 Sr-86 Sr-87 Sr-88, Calculating Average Atomic mass Worksheet-1 ( 1).docx A=(62.93x 69.2\100) + (64.93x 30.8\100) = 43.54 + 19.99 = 63.53 A = 63.53 amu 5.Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 u, 0.76% has a mass of 32.971u and 4.22% have a mass of 33.967u. (31.972x 95.00\100) + ( 32.971x 0.76 /100) + ( 33.967x 4.22/100) 30.3734 + 0.250 + 1.433 A= 32.056 amu 6. Calculating Average Atomic Mass Worksheet - Name ___Lisa ... - StuDocu The relative abundance and atomic masses are: 69% for mass of 62 30% for mass of 64. Calculate the average atomic mass of Copper. Show ALL work for full credit. 69/100 x 62 = 43. 30/100 x 64 = 19. ggghvhvhvhvhhv= 63-----Calculate the average atomic mass of Sulfur if: 95% of all Sulfur atoms have a mass of 31 u, 0% has a mass of 32 and 4% have a ... DOC Calculating Average Atomic Mass Worksheet Name______________________ 2. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 32, 0.76% has a mass of 33 and 4.22% have a mass of 34. 3. The four isotopes of lead are shown below, each with its percent by mass abundance and the composition of its nucleus. Using the following data, first calculate the atomic mass of each isotope.
PDF NAME Average Atomic Mass Worksheet: show all work. Magnesium consists of three naturally occurring isotopes. The percent abundance of these isotopes is as follows: 24Mg (78.70%), 25Mg (10.13%), and 26Mg (11.7%). The average atomic mass of the three isotopes is 24.3050 amu. If the atomic mass of 25Mg is 24.98584 amu, and 26Mg is 25.98259 amu, calculate the actual atomic mass of 24Mg. , DOCX Calculating Average Atomic Mass Worksheet Name______________________ The average atomic mass of silicon is 28.086amu. In general, what can we conclude about which isotope is the most abundant? 2. The four isotopes of lead are shown below, each with its percent by mass abundance and the composition of its nucleus. Using the following data, first calculate the mass number of each isotope. Then calculate the ... PDF Calculating Average Atomic Mass Worksheet Name - Solano Community College Calculating Average Atomic Mass Worksheet Name_____ 1. The term "average atomic mass" is a _____average, and so is calculated differently from a "normal" average. 2. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are 69.2% for a mass of 62.93amu and 30.8% for ... calculating percent abundance of isotopes worksheet Calculating Average Atomic Mass Worksheet Name. 9 Images about Calculating Average Atomic Mass Worksheet Name : Calculating Percent Abundance Of Isotopes Worksheet - worksheet, How To Calculate Percent Abundance Given Amu and also Calculating Average Atomic Mass Worksheet Name. Calculating Average Atomic Mass Worksheet Name, studylib.net,
average_atomic_mass (3).docx - Calculating Average Atomic... Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Isotopes of Silicon: Percent Abundance: Atomic Mass: Silicon-28 92.23% 27.97693 amu Silicon-29 4.68% 28.97649 amu Silicon-30 3.09% 29.97377 amu Calculate the average atomic mass for the three isotopes of Silicon.
PDF Chemistry: Average Atomic Mass Worksheet Chemistry: Average Atomic Mass Worksheet Calculate the average atomic mass for each element based on the natural abundance of its isotopes. 1. Find the average atomic mass for Li if 7.5% of Li atoms are 6Li with a mass of 6.0151223 amu and 92.5% are 7Li with a mass of 7.0160041 amu. 2.
PDF Answers Key for Unit Worksheets - livingston.org The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. X — amð 7) Magnesium consists of three naturally occurring isotopes. The percent abundance of these isotopes is as follows: 24 Mg (78.70%), 25Mg (10.13%), and 26Mg(11.7%). 'The average atomic mass of the three isotopes is 24.3050 amu.
DOC Calculating Average Atomic Mass Worksheet Name______________________ Calculate the average atomic mass of copper. 2. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% has a mass of 32.971amu and 4.22% have a mass of 33.967amu. 3. The three isotopes of lead are shown below, each with its percent by mass abundance and the composition of its nucleus.
Calculating Average Atomic Mass - Study.com Calculating Average Atomic Mass, High School Chemistry Skills Practice, 1. Carbon has three isotopes, namely Carbon-12, Carbon-13, and Carbon-14. C-12 has a mass of 12.000 amu and is 98.89%...
DOC Chemistry Worksheet - Livingston Public Schools The average atomic mass of silicon is 28.09amu. %29Si =, 4.7% mass = 29.4amu, Calculate the relative abundance of each isotope of iridium. The average atomic mass of iridium is 192.22amu, Isotope mass (u) relative abundance, Ir-191 191.0 ? 39.00%, Ir-193 193.0 ? 61.00%, Copper has two naturally occurring isotopes.
PDF Atoms and Isotopes Worksheet Atoms and Isotopes Worksheet Fill in the table with the correct information Isotope Isotope notation Atomic # Protons Electrons neutrons Oxygen-16 8 8 8 8 ... Calculate the average atomic mass of chlorine if its isotopes and % abundance are as follows. Show work. Mass of Isotope % abundance 36.96590 24.47 (.2447)(36.96590) = 9.04556 ...
PDF Average Atomic Mass Practice Problems - scott.kyschools.us Calculate the average atomic mass of chromium. (not in percents) , 7. The average atomic mass of copper is 63.55 amu. If the only two isotopes of copper have masses of 62.94 amu and 64.93 amu, what are the percentages of each? (Think algebra) , 8587, 8. Rubidium is a soft, silvery-white metal that has two common isotopes, Rb and Rb. , 8587,
How to Calculate Average Atomic Mass | Chemistry | Study.com Step 1: Identify the percentage of each isotope in the composition of the element and its mass. Coppe isotope A has 62.93 amu and composes 69.1% of natural copper. Copper isotope B has 64.93 amu...
DOCX Chemistry Worksheet Calculate the average atomic mass of gold with the 50% being gold-197 and 50% being gold-198. Calculate the average atomic mass of lithium, which occurs as two isotopes that have the following atomic masses and abundances in nature: 6.017 u, 7.30% and 7.018 u, 92.70%. Hydrogen is 99% 1H, 0.8% 2H, and 0.2% 3H. Calculate its average atomic mass.
PDF Isotope Practice Worksheet - Chemistry Calculate boron's atomic mass. Answer: 126.86 amu 11. Hydrogen is 99% 1H, 0.8% 2H, and 0.2% 3H. Calculate its average atomic mass. Answer: 1.21 amu 12. Rubidium is a soft, silvery-white metal that has two common isotopes, 85Rb and 87Rb. If the abundance of 85Rb is 80.2% and the abundance of 87Rb is 19.8%, what is the average atomic mass of ...
PDF Calculating Average Atomic Mass Worksheet - SI PROGRAM Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Atomic Mass: 27.97693 amu 28.97649 amu 29.97377 amu Isoto es of Silicon: Silicon-28 Silicon-29 Silicon-30 Percent Abundance: 92.23% 4.68% 3.09% Calculate the average atomic mass for the three isotopes of Silicon. (-9 Y(.o ( zq.q 7377) 0/0 2) Two isotopes Of ...
DOC Chemistry Worksheet - Forestville Central High School Calculate the average atomic mass of gold with the 50% being gold-197 and 50% being gold-198. Calculate the average atomic mass of lithium, which occurs as two isotopes that have the following atomic masses and abundances in nature: 6.017 u, 7.30% and 7.018 u, 92.70%. Hydrogen is 99% 1H, 0.8% 2H, and 0.2% 3H. Calculate its average atomic mass.
Chemistry: Average Atomic Mass Worksheet Flashcards | Quizlet Find the average atomic mass for Cl is 75.78% of Cl atoms are 35Cl with a mass of 34.96885271 amu and 24.22% are 37Cl with a mass of 36.96590260 amu. Find the average atomic mass for Mg if 78.99% of Mg atoms are 24Mg with a mass of 23.9850419 amu, 10.00% are 25Mg with a mass of 24.9858370 amu, and 11.01% are 26Mg with a mass of 25.9825930 amu.



























0 Response to "43 calculating average atomic mass worksheet"
Post a Comment