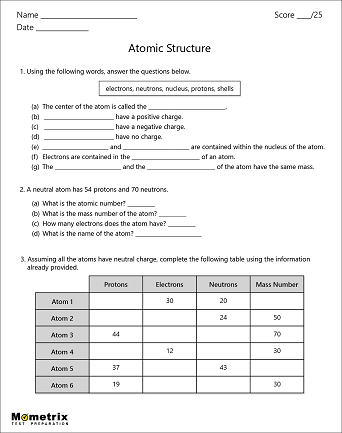
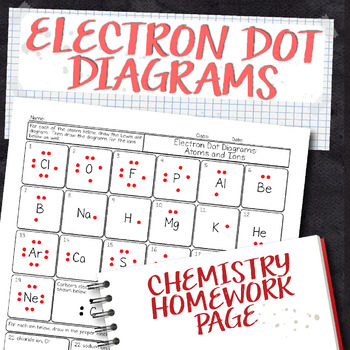
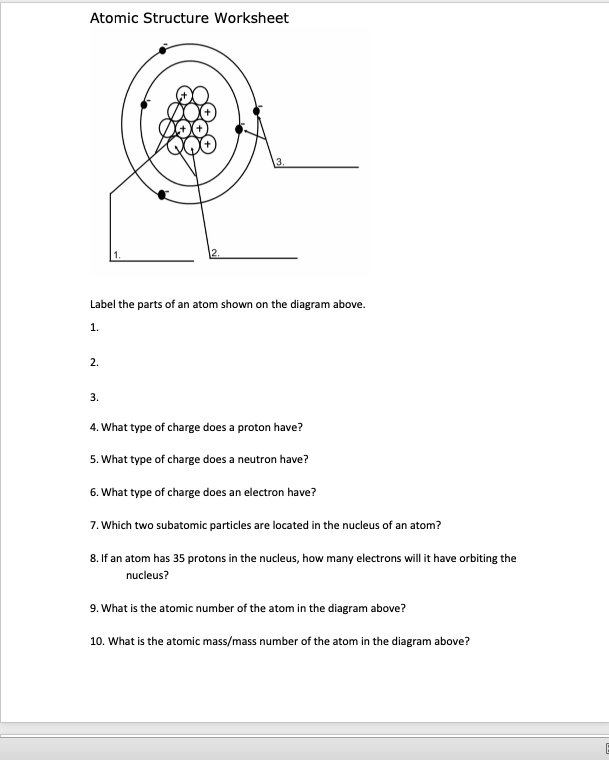
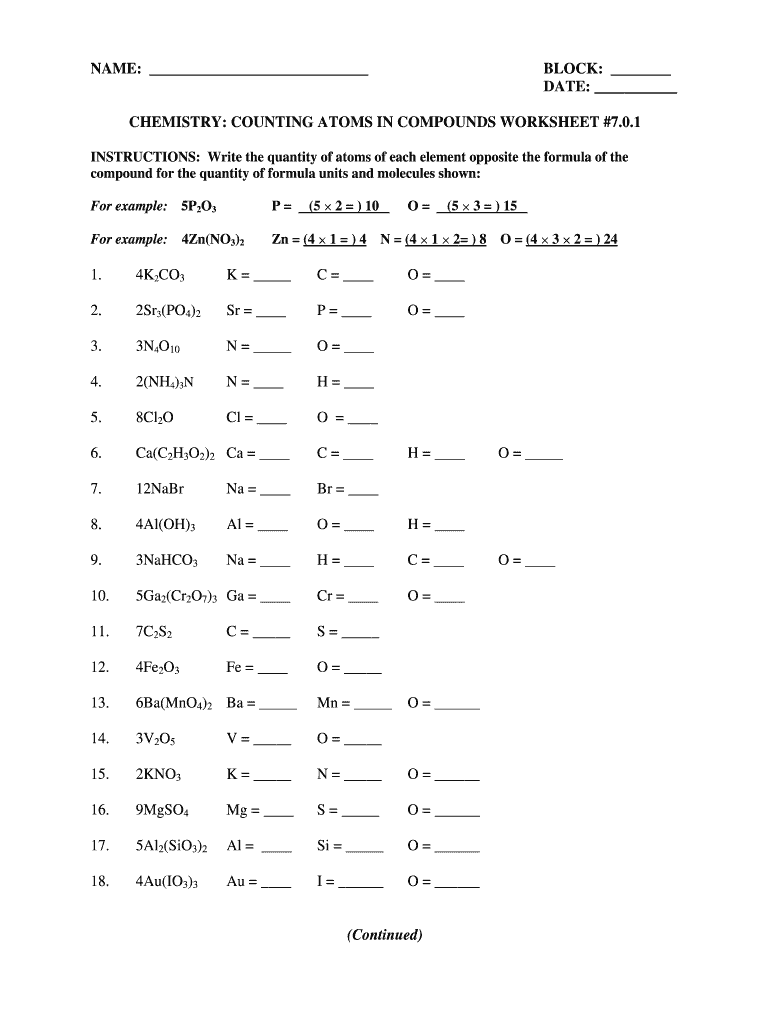
45 worksheet electrons in atoms
electron | Definition, Mass, & Facts | Britannica 22/09/2022 · electron, lightest stable subatomic particle known. It carries a negative charge of 1.602176634 × 10−19 coulomb, which is considered the basic unit of electric charge. The rest mass of the electron is 9.1093837015 × 10−31 kg, which is only 11,836the mass of a proton. An electron is therefore considered nearly massless in comparison with a proton or a neutron, … qrg.northwestern.edu › projects › vssWhat is an atom? - Qualitative Reasoning Group An atom a fundamental piece of matter. (Matter is anything that can be touched physically.) Everything in the universe (except energy) is made of matter, and, so, everything in the universe is made of atoms. An atom itself is made up of three tiny kinds of particles called subatomic particles: protons, neutrons, and electrons.
› atoms-moleculesAtoms and Molecules Worksheets - Math Worksheets 4 Kids The basic building block of all matter, atoms are at the core of all that we see, touch, smell, feel, and taste. Molecules are formed when two or more atoms link up. A reasonable knowledge of atoms and molecules is indispensable for us to demystify the universe.

Worksheet electrons in atoms
Build an Atom - Atoms | Atomic Structure | Isotope Symbols Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! Then play a game to test your ideas! Skip to Main Content › science › physicalAtom Worksheets Atom is the most basic unit of matter. It has a dense nucleus with a cloud of negatively charged electrons surrounding it. Here are the parts of an Atom: Electron; it is a subatomic particle with a negative electrical charge. The mass of an electron is so small that it is generally not even considered. Basic Atomic Structure Worksheet Key - Neshaminy School District Give the symbol of and the number of electrons in a neutral atom of: Uranium Boron Chlorine Iodine Xenon Give the symbol of and the number of neutrons in one atom of: (Mass numbers are ALWAYS whole numbers...show your calculations) Barium Carbon Fluorine Europium IS-I 10 Bismuth Hydrogen Magnesium Mercury
Worksheet electrons in atoms. › bitesize › guidesElectrolysis of molten salts - Electrolysis - AQA - GCSE ... Here, they lose electrons to form chlorine atoms. The atoms join up in pairs to form Cl 2 molecules, so chlorine gas is formed at the positive electrode. During the electrolysis of molten salts, a ... Chem4Kids.com: Atoms Protons, neutrons, and electrons can then organize to form atoms. Atoms are then used to create the molecules around us. As we just learned, there are almost 120 elements that can be found in the molecules we know. Smaller molecules can work together and build macromolecules. It just goes on. Everything you see or imagine is built from something else. You could start … › science › the_atomScience for Kids: The Atom - Ducksters Atoms last a long time, in most cases forever. They can change and undergo chemical reactions, sharing electrons with other atoms. But the nucleus is very hard to split, meaning most atoms are around for a long time. Structure of the Atom At the center of the atom is the nucleus. The nucleus is made up of the protons and neutrons. › worksheets › atomicAtomic Structure Worksheet - Basic Electricity Most helium atoms contain 2 neutrons, but some may contain more or less than 2. Each atom of aluminum is guaranteed to contain 13 protons. Unless the atom is electrically charged, it will contain 13 electrons as well to balance the charge of the protons. Most aluminum atoms contain 14 neutrons, but some may contain more or less than 14.
› Protons, Neutrons, and ElectronsProtons, Neutrons, and Electrons Practice Worksheet - SMATCOE Protons, Neutrons, and Electrons Practice Worksheet Calculating the number of each particle in an atom: # Protons = Atomic Number # Electrons = Protons # Neutrons = Atomic Mass – Atomic Number OR Big # - Small # Use the periodic table to find the numbers of protons, neutrons, and electrons for atoms of the following elements. Name of Element Isotope Worksheet Answer Key - ISD 622 # of electrons mass # a35 33 # of protons 19 # of neutrons 22 Isoto e name uranium-235 uranium-23 8 boron- 10 boron-11 atomic # Phos hocus-33 15 Write the hyphen notation and the nuclide (nuclear) symbol for an isotope that has 17 protons, 17 electrons, and 20 neutrons. 37 Isotopes are atoms of the same element with a different number of Loading... - BrainPop Loading... - BrainPop ... Loading... Basic Atomic Structure Worksheet Key - Neshaminy School District Give the symbol of and the number of electrons in a neutral atom of: Uranium Boron Chlorine Iodine Xenon Give the symbol of and the number of neutrons in one atom of: (Mass numbers are ALWAYS whole numbers...show your calculations) Barium Carbon Fluorine Europium IS-I 10 Bismuth Hydrogen Magnesium Mercury
› science › physicalAtom Worksheets Atom is the most basic unit of matter. It has a dense nucleus with a cloud of negatively charged electrons surrounding it. Here are the parts of an Atom: Electron; it is a subatomic particle with a negative electrical charge. The mass of an electron is so small that it is generally not even considered. Build an Atom - Atoms | Atomic Structure | Isotope Symbols Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! Then play a game to test your ideas! Skip to Main Content





































0 Response to "45 worksheet electrons in atoms"
Post a Comment