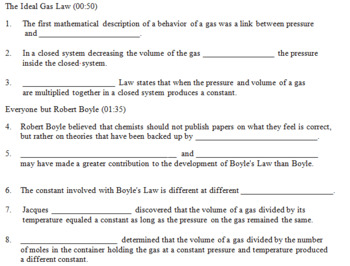
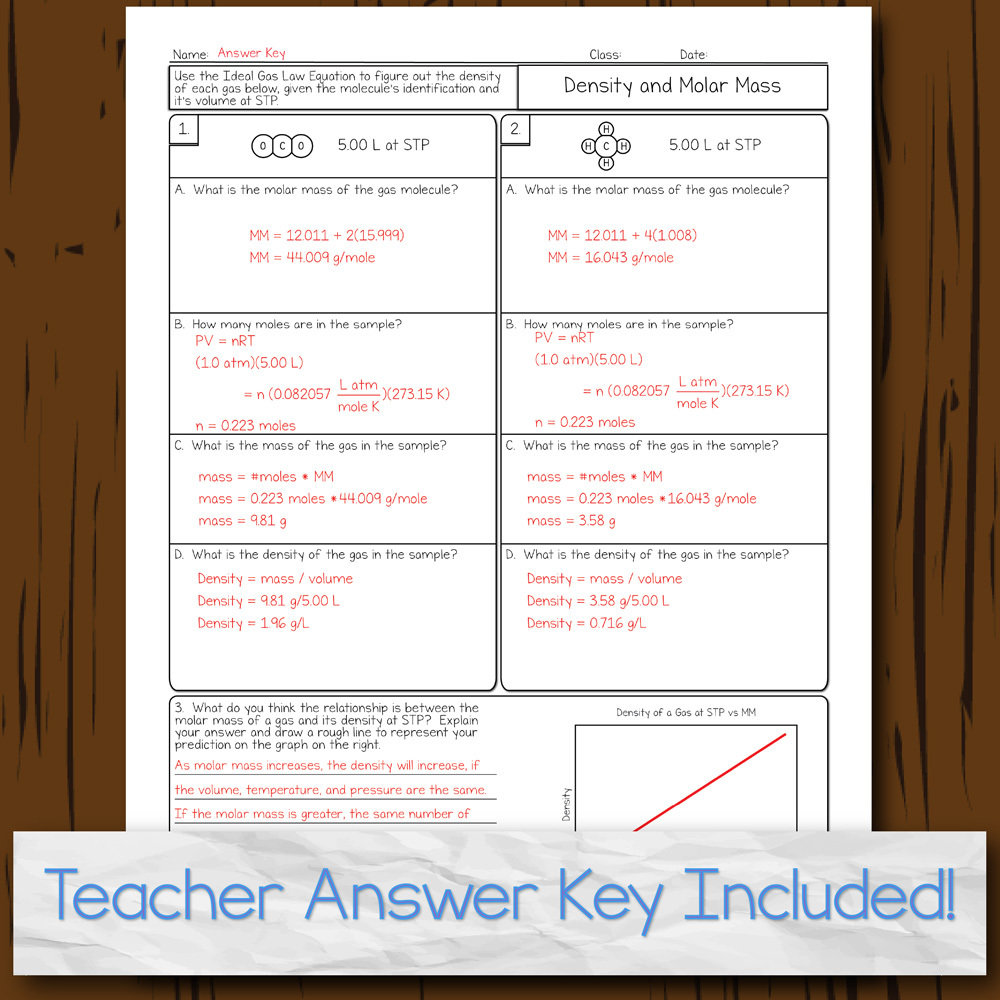
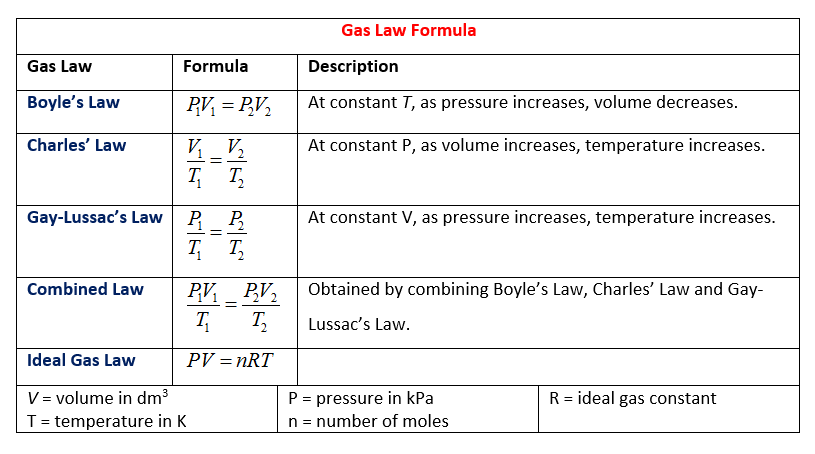
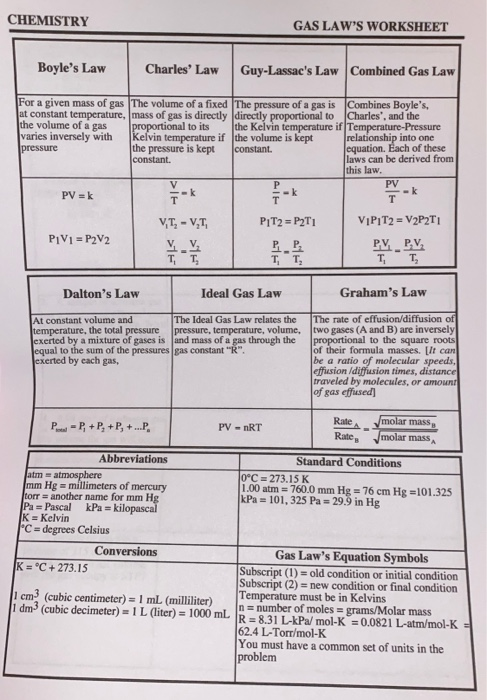
44 chemistry the ideal gas law worksheet
STEM Success Center CHEM 150: Ch. 10 Ideal Gas Law ... - CSUSM CHEM 150. Worksheet. CHEM 150: Ch. 10 Ideal Gas Law. 1. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 L? Ideal Gas Law Worksheet - StuDocu ideal gas law practice worksheet solve the following problems using the ideal gas law: how many moles of gas does it take to occupy 120 liters at pressure ...
Mixed Gas Laws Worksheet - Everett Community College Mixed Gas Laws Worksheet. 1). How many moles of gas occupy 98 L at a pressure of 2.8 atmospheres and a temperature of 292 K?

Chemistry the ideal gas law worksheet
Ideal Gas Law Worksheet PV = nRT - supertallteacher AP CHEM Gas Laws Worksheet. Use the ideal gas law, PV= nRT and the universal gas constants: R = 62.36 L*torr or. R= 0.08206 L*atm. Ideal Gas Law Worksheet Period ______. Given: Ideal Gas Law = then P = n = V = T = R = What pressure is required to contain 0.023 moles of nitrogen gas in a 4.2 L container at a. Ideal Gas Law Worksheet PV = nRT ... Gas Law Worksheet PV = nRT. Use the ideal gas law, “PerV-nRT”, and the universal gas constant R = 0.0821 L*atm to solve the following problems:.
Chemistry the ideal gas law worksheet. ideal gas law - School District of Clayton How many moles of oxygen will occupy a volume of 2.5 liters at 1.2 atm and 25° C? 12 mol. PV. PV=DRT n= RT. 1.2.2.5. ·0821.298. Worksheet 7 - Ideal Gas Law To standardize results, chemists often use a set of experimental conditions, called standard temperature and pressure (STP). a). Standard pressure = __1___ atm ... Gas volumes and the Ideal gas law worksheet - Liveworksheets.com ID: 1834305. Language: English School subject: Chemistry Grade/level: 10. Age: 14-16. Main content: Gas volumes. Other contents: Add to my workbooks (8) The Ideal Gas Law - Chemistry Chemistry: The Ideal Gas Law ... At –45oC, 71 g of fluorine gas take up 6843 mL of space. What is the pressure of the gas, in kPa?
Ideal Gas Law Worksheet PV = nRT ... Gas Law Worksheet PV = nRT. Use the ideal gas law, “PerV-nRT”, and the universal gas constant R = 0.0821 L*atm to solve the following problems:. Ideal Gas Law Worksheet Period ______. Given: Ideal Gas Law = then P = n = V = T = R = What pressure is required to contain 0.023 moles of nitrogen gas in a 4.2 L container at a. Ideal Gas Law Worksheet PV = nRT - supertallteacher AP CHEM Gas Laws Worksheet. Use the ideal gas law, PV= nRT and the universal gas constants: R = 62.36 L*torr or. R= 0.08206 L*atm.

.jpg?revision=1)

























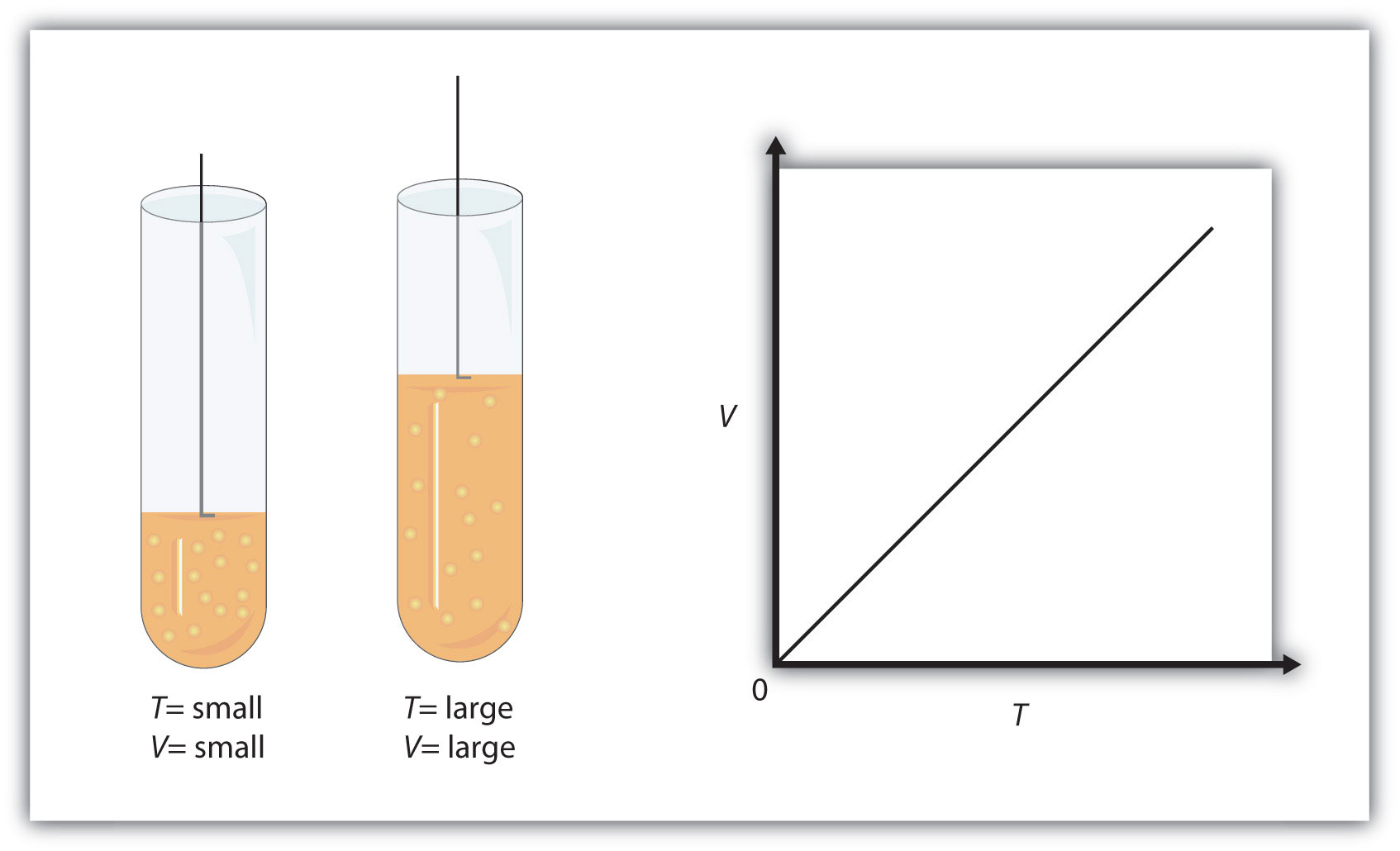



0 Response to "44 chemistry the ideal gas law worksheet"
Post a Comment